The signal recognition particle (SRP) RNA links conformational changes in the SRP to protein targeting
- PMID: 17507650
- PMCID: PMC1924838
- DOI: 10.1091/mbc.e07-02-0117
The signal recognition particle (SRP) RNA links conformational changes in the SRP to protein targeting
Abstract
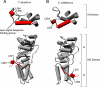
The RNA component of the signal recognition particle (SRP) is universally required for cotranslational protein targeting. Biochemical studies have shown that SRP RNA participates in the central step of protein targeting by catalyzing the interaction of the SRP with the SRP receptor (SR). SRP RNA also accelerates GTP hydrolysis in the SRP.SR complex once formed. Using a reverse-genetic and biochemical analysis, we identified mutations in the E. coli SRP protein, Ffh, that abrogate the activity of the SRP RNA and cause corresponding targeting defects in vivo. The mutations in Ffh that disrupt SRP RNA activity map to regions that undergo dramatic conformational changes during the targeting reaction, suggesting that the activity of the SRP RNA is linked to the major conformational changes in the signal sequence-binding subunit of the SRP. In this way, the SRP RNA may coordinate the interaction of the SRP and the SR with ribosome recruitment and transfer to the translocon, explaining why the SRP RNA is an indispensable component of the protein targeting machinery.
Figures

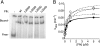



Similar articles
-
RNA-mediated interaction between the peptide-binding and GTPase domains of the signal recognition particle.Nat Struct Mol Biol. 2005 Dec;12(12):1116-22. doi: 10.1038/nsmb1025. Epub 2005 Nov 20. Nat Struct Mol Biol. 2005. PMID: 16299512
-
SRP RNA provides the physiologically essential GTPase activation function in cotranslational protein targeting.RNA. 2007 Feb;13(2):240-50. doi: 10.1261/rna.135407. Epub 2006 Dec 12. RNA. 2007. PMID: 17164479 Free PMC article.
-
The crystal structure of the signal recognition particle in complex with its receptor.Science. 2011 Feb 18;331(6019):881-6. doi: 10.1126/science.1196473. Science. 2011. PMID: 21330537 Free PMC article.
-
Structural insights into the signal recognition particle.Annu Rev Biochem. 2004;73:539-57. doi: 10.1146/annurev.biochem.73.011303.074048. Annu Rev Biochem. 2004. PMID: 15189152 Review.
-
Co-translational protein targeting by the signal recognition particle.FEBS Lett. 2005 Feb 7;579(4):921-6. doi: 10.1016/j.febslet.2004.11.049. FEBS Lett. 2005. PMID: 15680975 Review.
Cited by
-
The very early evolution of protein translocation across membranes.PLoS Comput Biol. 2021 Mar 8;17(3):e1008623. doi: 10.1371/journal.pcbi.1008623. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33684113 Free PMC article.
-
The 4.5S RNA component of the signal recognition particle is required for group A Streptococcus virulence.Microbiology (Reading). 2010 May;156(Pt 5):1342-1350. doi: 10.1099/mic.0.036558-0. Epub 2010 Jan 28. Microbiology (Reading). 2010. PMID: 20110295 Free PMC article.
-
Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation.Chem Rev. 2018 Feb 28;118(4):1691-1741. doi: 10.1021/acs.chemrev.7b00305. Epub 2018 Jan 10. Chem Rev. 2018. PMID: 29319301 Free PMC article. Review.
-
SRP RNA controls a conformational switch regulating the SRP-SRP receptor interaction.Nat Struct Mol Biol. 2008 Sep;15(9):916-23. doi: 10.1038/nsmb.1467. Nat Struct Mol Biol. 2008. PMID: 19172744 Free PMC article.
-
The Malignant Role of Exosomes as Nanocarriers of Rare RNA Species.Int J Mol Sci. 2020 Aug 15;21(16):5866. doi: 10.3390/ijms21165866. Int J Mol Sci. 2020. PMID: 32824183 Free PMC article. Review.
References
-
- Batey R. T., Rambo R. P., Lucast L., Rha B., Doudna J. A. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000;287:1232–1239. - PubMed
-
- Buskiewicz I., Peske F., Wieden H. J., Gryczynski I., Rodnina M. V., Wintermeyer W. Conformations of the signal recognition particle protein Ffh from Escherichia coli as determined by FRET. J. Mol. Biol. 2005b;351:417–430. - PubMed
-
- Connolly T., Rapiejko P. J., Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991;252:1171–1173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

