Erythroid-specific expression of beta-globin by the sleeping beauty transposon for Sickle cell disease
- PMID: 17508724
- PMCID: PMC3893920
- DOI: 10.1021/bi6024484
Erythroid-specific expression of beta-globin by the sleeping beauty transposon for Sickle cell disease
Abstract
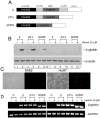
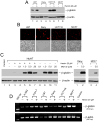
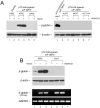
Sickle cell disease (SCD) results predominately from a single monogenic mutation that affects thousands of individuals worldwide. Gene therapy approaches have focused on using viral vectors to transfer wild-type beta- or gamma-globin transgenes into hematopoietic stem cells for long-term expression of the recombinant globins. In this study, we investigated the use of a novel nonviral vector system, the Sleeping Beauty (SB) transposon (Tn) to insert a wild-type beta-globin expression cassette into the human genome for sustained expression of beta-globin. We initially constructed a beta-globin expression vector composed of the hybrid cytomegalovirus (CMV) enhancer chicken beta-actin promoter (CAGGS) and full-length beta-globin cDNA, as well as truncated forms lacking either the 3' or 3' and 5' untranslated regions (UTRs), to optimize expression of beta-globin. Beta-globin with its 5' UTR was efficiently expressed from its cDNA in K-562 cells induced with hemin. However, expression was constitutive and not erythroid-specific. We then constructed cis SB-Tn-beta-globin plasmids using a minimal beta-globin gene driven by hybrid promoter IHK (human ALAS2 intron 8 erythroid-specific enhancer, HS40 core element from human alphaLCR, ankyrin-1 promoter), IHbetap (human ALAS2 intron 8 erythroid-specific enhancer, HS40 core element from human alphaLCR, beta-globin promoter), or HS3betap (HS3 core element from human betaLCR, beta-globin promoter) to establish erythroid-specific expression of beta-globin. Stable genomic insertion of the minimal gene and expression of the beta-globin transgene for >5 months at a level comparable to that of the endogenous gamma-globin gene were achieved using a SB-Tn beta-globin cis construct. Interestingly, erythroid-specific expression of beta-globin driven by IHK was regulated primarily at the translational level, in contrast to post-transcriptional regulation in non-erythroid cells. The SB-Tn system is a promising nonviral vector for efficient genomic insertion conferring stable, persistent erythroid-specific expression of beta-globin.
Figures





Similar articles
-
Erythroid-specific expression of β-globin from Sleeping Beauty-transduced human hematopoietic progenitor cells.PLoS One. 2011;6(12):e29110. doi: 10.1371/journal.pone.0029110. Epub 2011 Dec 28. PLoS One. 2011. PMID: 22216176 Free PMC article.
-
Optimization of recombinant adeno-associated viral vectors for human beta-globin gene transfer and transgene expression.Hum Gene Ther. 2008 Apr;19(4):365-75. doi: 10.1089/hum.2007.173. Hum Gene Ther. 2008. PMID: 18399730
-
Adeno-associated virus 2-mediated transduction and erythroid lineage-restricted expression from parvovirus B19p6 promoter in primary human hematopoietic progenitor cells.J Hematother Stem Cell Res. 1999 Dec;8(6):585-92. doi: 10.1089/152581699319740. J Hematother Stem Cell Res. 1999. PMID: 10645765
-
Gene transfer. A potential approach to gene therapy for sickle cell disease.Ann N Y Acad Sci. 1989;565:37-43. doi: 10.1111/j.1749-6632.1989.tb24147.x. Ann N Y Acad Sci. 1989. PMID: 2672970 Review.
-
Molecular analysis of erythropoiesis. A current appraisal.Exp Cell Res. 1984 Dec;155(2):321-44. doi: 10.1016/0014-4827(84)90194-0. Exp Cell Res. 1984. PMID: 6389159 Review.
Cited by
-
Nanocapsule-delivered Sleeping Beauty mediates therapeutic Factor VIII expression in liver sinusoidal endothelial cells of hemophilia A mice.J Clin Invest. 2009 Jul;119(7):2086-99. doi: 10.1172/JCI34332. Epub 2009 Jun 8. J Clin Invest. 2009. PMID: 19509468 Free PMC article.
-
Long-term reduction of jaundice in Gunn rats by nonviral liver-targeted delivery of Sleeping Beauty transposon.Hepatology. 2009 Sep;50(3):815-24. doi: 10.1002/hep.23060. Hepatology. 2009. PMID: 19585550 Free PMC article.
-
β-Globin sleeping beauty transposon reduces red blood cell sickling in a patient-derived CD34(+)-based in vitro model.PLoS One. 2013 Nov 18;8(11):e80403. doi: 10.1371/journal.pone.0080403. eCollection 2013. PLoS One. 2013. PMID: 24260386 Free PMC article.
-
The expanding universe of transposon technologies for gene and cell engineering.Mob DNA. 2010 Dec 7;1(1):25. doi: 10.1186/1759-8753-1-25. Mob DNA. 2010. PMID: 21138556 Free PMC article.
-
A human/murine chimeric fab antibody neutralizes anthrax lethal toxin in vitro.Clin Dev Immunol. 2013;2013:475809. doi: 10.1155/2013/475809. Epub 2013 Jun 5. Clin Dev Immunol. 2013. PMID: 23861692 Free PMC article.
References
-
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. - PubMed
-
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, Acharya SA, Ellis J, London IM, Eaves CJ, Humphries RK, Beuzard Y, Nagel RL, Leboulch P. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. - PubMed
-
- Mohamedali A, Moreau-Gaudry F, Richard E, Xia P, Nolta J, Malik p. Self-inactivating lentiviral vectors resist proviral methylation but do not confer position-independent expression in hematopoietic stem cells. Mol Ther. 2004;10:249–259. - PubMed
-
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. - PubMed
-
- Moreau-Gaudry F, Xia P, Jiang G, Perelman NP, Bauer G, Ellis J, Surinya KH, Mavilio F, Shen CK, Malik P. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood. 2001;98:2664–2672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

