The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine
- PMID: 17509035
- PMCID: PMC2203270
- DOI: 10.1111/j.1365-2125.2007.02944.x
The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine
Abstract
Aims: To evaluate the effects of three ABCG2 variants (Q141K, V12M and Q126X), which are known to have altered transport properties in vitro, on the disposition of lamivudine in healthy subjects.
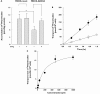
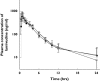
Methods: To evaluate whether lamivudine is a substrate of ABCG2, intracellular accumulation and vectorial transport of 3H-lamivudine were determined in MDCK-ABCG2 cells. The pharmacokinetic parameters of lamivudine were compared among subjects with four different ABCG2 genotypes, including wild type (seven subjects), K141/K141 (six subjects), Q126/Stop126 (four subjects) and M12/M12 (five subjects) after a single oral dose of 100 mg lamivudine.
Results: The intracellular accumulation of lamivudine in MDCK-ABCG2 cells was significantly lower than that in MDCK-mock cells, but fumitremorgin C reversed the intracellular lamivudine concentration to that of MDCK-mock cells. The ABCG2-mediated transport of lamivudine was saturable and the values of Km and Vmax were 216.5 +/- 58 microm and 20.42 +/- 2.9 nmol h(-1) per 10(6) cells, respectively. After lamivudine administration to healthy subjects, the AUC of lamivudine showed no difference among subjects with different ABCG2 genotypes; 2480 +/- 502, 2207 +/- 1019, 2422 +/- 239, 2552 +/- 698 ng h(-1) ml(-1) for wild type, K141/K141, Q126/Stop126 and M12/M12 genotype, respectively (P = 0.85). The estimated 95% confidence intervals for the mean difference between K141/K141, Q126/Stop126, M12/M12 and wild as reference were (-1053, 507), (-555, 439) and (-552, 696), respectively. No other pharmacokinetic parameters were estimated to be significantly different among four different ABCG2 genotypes tested.
Conclusions: Lamivudine appeared to be a substrate of ABCG2 in vitro, but the disposition of lamivudine was not significantly influenced by known in vitro functional variants of ABCG2, Q141K, V12M and Q126X in healthy subjects.
Figures
 ) variant of ABCG2
) variant of ABCG2
Similar articles
-
Single nucleotide polymorphisms modify the transporter activity of ABCG2.Cancer Chemother Pharmacol. 2005 Aug;56(2):161-72. doi: 10.1007/s00280-004-0931-x. Epub 2005 Apr 19. Cancer Chemother Pharmacol. 2005. PMID: 15838659
-
Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2.Int J Cancer. 2004 Mar 20;109(2):238-46. doi: 10.1002/ijc.11669. Int J Cancer. 2004. PMID: 14750175
-
ABCG2/BCRP dysfunction as a major cause of gout.Nucleosides Nucleotides Nucleic Acids. 2011 Dec;30(12):1117-28. doi: 10.1080/15257770.2011.633954. Nucleosides Nucleotides Nucleic Acids. 2011. PMID: 22132966
-
[Recent progress and prospects for research on urate efflux transporter ABCG2].Nihon Rinsho. 2014 Apr;72(4):757-65. Nihon Rinsho. 2014. PMID: 24796111 Review. Japanese.
-
Effect of the ATP-binding cassette transporter ABCG2 on pharmacokinetics: experimental findings and clinical implications.Expert Opin Drug Metab Toxicol. 2013 Mar;9(3):287-306. doi: 10.1517/17425255.2013.742063. Epub 2013 Jan 7. Expert Opin Drug Metab Toxicol. 2013. PMID: 23289909 Review.
Cited by
-
ABCB1 variation and treatment response in AIDS patients: initial results of the Henan cohort.PLoS One. 2013;8(1):e55197. doi: 10.1371/journal.pone.0055197. Epub 2013 Jan 25. PLoS One. 2013. PMID: 23372834 Free PMC article.
-
Indinavir Alters the Pharmacokinetics of Lamivudine Partially via Inhibition of Multidrug and Toxin Extrusion Protein 1 (MATE1).Pharm Res. 2018 Jan 4;35(1):14. doi: 10.1007/s11095-017-2290-4. Pharm Res. 2018. PMID: 29302757
-
Clinically relevant mutations in the ABCG2 transporter uncovered by genetic analysis linked to erythrocyte membrane protein expression.Sci Rep. 2018 May 10;8(1):7487. doi: 10.1038/s41598-018-25695-z. Sci Rep. 2018. PMID: 29749379 Free PMC article.
-
Racial Disparity in Drug Disposition in the Digestive Tract.Int J Mol Sci. 2021 Jan 21;22(3):1038. doi: 10.3390/ijms22031038. Int J Mol Sci. 2021. PMID: 33494365 Free PMC article. Review.
-
Prediction of Drug Transfer into Milk Considering Breast Cancer Resistance Protein (BCRP)-Mediated Transport.Pharm Res. 2015 Aug;32(8):2527-37. doi: 10.1007/s11095-015-1641-2. Epub 2015 Feb 19. Pharm Res. 2015. PMID: 25690342
References
-
- Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. - PubMed
-
- Lockhart AC, Tirona RG, Kim RB. Pharmacogenetics of ATP-binding cassette transporters in cancer and chemotherapy. Mol Cancer Ther. 2003;2:685–98. - PubMed
-
- Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist Updat. 2003;6:71–84. - PubMed
-
- Allen JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2) Mol Cancer Ther. 2002;1:427–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

