Immunogenicity of the outer domain of a HIV-1 clade C gp120
- PMID: 17509143
- PMCID: PMC1891314
- DOI: 10.1186/1742-4690-4-33
Immunogenicity of the outer domain of a HIV-1 clade C gp120
Abstract
Background: The possibility that a sub domain of a C clade HIV-1 gp120 could act as an effective immunogen was investigated. To do this, the outer domain (OD) of gp120CN54 was expressed and characterized in a construct marked by a re-introduced conformational epitope for MAb 2G12. The expressed sequence showed efficient epitope retention on the isolated ODCN54 suggesting authentic folding. To facilitate purification and subsequent immunogenicity ODCN54 was fused to the Fc domain of human IgG1. Mice were immunised with the resulting fusion proteins and also with gp120CN54-Fc and gp120 alone.
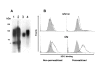
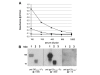
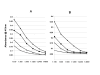
Results: Fusion to Fc was found to stimulate antibody titre and Fc tagged ODCN54 was substantially more immunogenic than non-tagged gp120. Immunogenicity appeared the result of Fc facilitated antigen processing as immunisation with an Fc domain mutant that reduced binding to the FcR lead to a reduction in antibody titre when compared to the parental sequence. The breadth of the antibody response was assessed by serum reaction with five overlapping fragments of gp120CN54 expressed as GST fusion proteins in bacteria. A predominant anti-inner domain and anti-V3C3 response was observed following immunisation with gp120CN54-Fc and an anti-V3C3 response to the ODCN54-Fc fusion.
Conclusion: The outer domain of gp120CN54 is correctly folded following expression as a C terminal fusion protein. Immunogenicity is substantial when targeted to antigen presenting cells but shows V3 dominance in the polyvalent response. The gp120 outer domain has potential as a candidate vaccine component.
Figures



References
-
- Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

