Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development
- PMID: 17509542
- PMCID: PMC1994791
- DOI: 10.1016/j.brainres.2007.04.043
Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development
Abstract
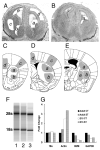
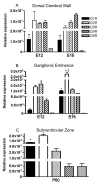
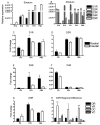
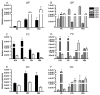
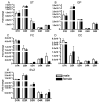
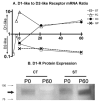
The outcome of dopaminergic signaling and effectiveness of dopaminergic drugs depend on the relative preponderance of each of the five dopamine receptors in a given brain region. The separate contribution of each receptor to overall dopaminergic tone is difficult to establish at a functional level due to lack of receptor subtype specific pharmacological agents. A surrogate for receptor function is receptor protein or mRNA expression. We examined dopamine receptor mRNA expression by quantitative reverse transcription real-time PCR in the striatum, globus pallidus, frontal cortex and cingulate cortex of embryonic and postnatal mice. Samples of each region were collected by laser capture microdissection. D1- and D2-receptor mRNAs were the most abundant in all the regions of the mature brain. The D1-receptor was predominant over the D2-receptor in the frontal and cingulate cortices whereas the situation was reversed in the striatum and globus pallidus. In the proliferative domains of the embryonic forebrain, D3-, D4- and D5-receptors were predominant. In the corpus striatum and cerebral cortex, the D3- and D4-receptors were the only receptors that showed marked developmental regulation. By analyzing D1 receptor protein expression, we show that developmental changes in mRNA expression reliably translate into changes in protein levels, at least for the D1-receptor.
Figures

References
-
- Altman J, Bayer SA. Atlas of prenatal rat brain development. CRC Press; Boca raton, Fl., USA: 1995.
-
- Bayer SA. Neurogenesis in the rat neostriatum. International Journal of Developmental Neuroscience. 1984;2:163–175. - PubMed
-
- Cepeda C, Levine MS. Dopamine and N-Methyl-D-Aspartate Receptor Interactions in the Neostriatum. Dev Neurosci. 1998;20:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

