Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis
- PMID: 17510284
- PMCID: PMC1877747
- DOI: 10.1101/gad.1546107
Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis
Abstract
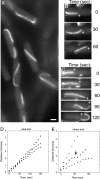
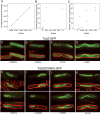
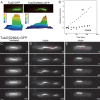
Prokaryotes rely on a distant tubulin homolog, FtsZ, for assembling the cytokinetic ring essential for cell division, but are otherwise generally thought to lack tubulin-like polymers that participate in processes such as DNA segregation. Here we characterize a protein (TubZ) from the Bacillus thuringiensis virulence plasmid pBtoxis, which is a member of the tubulin/FtsZ GTPase superfamily but is only distantly related to both FtsZ and tubulin. TubZ assembles dynamic, linear polymers that exhibit directional polymerization with plus and minus ends, movement by treadmilling, and a critical concentration for assembly. A point mutation (D269A) that alters a highly conserved catalytic residue within the T7 loop completely eliminates treadmilling and allows the formation of stable polymers at a much lower protein concentration than the wild-type protein. When expressed in trans, TubZ(D269A) coassembles with wild-type TubZ and significantly reduces the stability of pBtoxis, demonstrating a direct correlation between TubZ dynamics and plasmid maintenance. The tubZ gene is in an operon with tubR, which encodes a putative DNA-binding protein that regulates TubZ levels. Our results suggest that TubZ is representative of a novel class of prokaryotic cytoskeletal proteins important for plasmid stability that diverged long ago from the ancient tubulin/FtsZ ancestor.
Figures





References
-
- Adachi S., Hori K., Hiraga S., Hori K., Hiraga S., Hiraga S. Subcellular positioning of F plasmid mediated by dynamic localization of SopA and SopB. J. Mol. Biol. 2006;356:850–863. - PubMed
-
- Addinall S.G., Holland B., Holland B. The tubulin ancestor, FtsZ, draughtsman, designer and driving force for bacterial cytokinesis. J. Mol. Biol. 2002;318:219–236. - PubMed
-
- Amos L.A., van den Ent F., Lowe J., van den Ent F., Lowe J., Lowe J. Structural/functional homology between the bacterial and eukaryotic cytoskeletons. Curr. Opin. Cell Biol. 2004;16:24–31. - PubMed
-
- Becker E., Herrera N.C., Gunderson F.Q., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Herrera N.C., Gunderson F.Q., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Gunderson F.Q., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Derman A.I., Dance A.L., Sims J., Larsen R.A., Pogliano J., Dance A.L., Sims J., Larsen R.A., Pogliano J., Sims J., Larsen R.A., Pogliano J., Larsen R.A., Pogliano J., Pogliano J. DNA segregation by the bacterial actin AlfA during Bacillus subtilis growth and development. EMBO J. 2006;25:5919–5931. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
