The SOCS box of suppressor of cytokine signaling-3 contributes to the control of G-CSF responsiveness in vivo
- PMID: 17510322
- PMCID: PMC1975836
- DOI: 10.1182/blood-2007-03-079178
The SOCS box of suppressor of cytokine signaling-3 contributes to the control of G-CSF responsiveness in vivo
Abstract
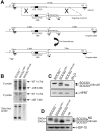
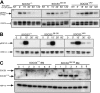
Suppressor of cytokine signaling 3 (SOCS3) is a negative regulator of granulocyte-colony stimulating factor (G-CSF) signaling in vivo. SOCS proteins regulate cytokine signaling by binding, via their SH2 domains, to activated cytokine receptors or their associated Janus kinases. In addition, they bind to the elongin B/C ubiquitin ligase complex via the SOCS box. To ascertain the contribution of the SOCS box of SOCS3 to in vivo regulation of G-CSF signaling, we generated mice expressing a truncated SOCS3 protein lacking the C-terminal SOCS box (SOCS3(Delta SB/Delta SB)). SOCS3(Delta SB/Delta SB) mice were viable, had normal steady-state hematopoiesis, and did not develop inflammatory disease. Despite the mild phenotype, STAT3 activation in response to G-CSF signaling was prolonged in SOCS3(Delta SB/Delta SB) bone marrow. SOCS3(Delta SB/Delta SB) bone marrow contained increased numbers of colony-forming cells responsive to G-CSF and IL-6. Treatment of the mice with pharmacologic doses of G-CSF, which mimics emergency granulopoiesis and therapeutic use of G-CSF, revealed that SOCS3(Delta SB/Delta SB) mice were hyperresponsive to G-CSF. Compared with wild-type mice, SOCS3(Delta SB/Delta SB) mice developed a more florid arthritis when tested using an acute disease model. Overall, the results establish a role for the SOCS box of SOCS3 in the in vivo regulation of G-CSF signaling and the response to inflammatory stimuli.
Figures
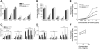



References
-
- Roberts AW. G-CSF: a key regulator of neutrophil production, but that's not all! Growth Factors. 2005;23:33–41. - PubMed
-
- Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–920. - PubMed
-
- Naka T, Narazaki M, Hirate M, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. - PubMed
-
- Endo TA, Masuhara M, Yokochi M, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. - PubMed
-
- Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

