Conserved roles of Sam50 and metaxins in VDAC biogenesis
- PMID: 17510655
- PMCID: PMC2002532
- DOI: 10.1038/sj.embor.7400982
Conserved roles of Sam50 and metaxins in VDAC biogenesis
Abstract
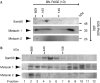
Voltage-dependent anion-selective channel (VDAC) is a beta-barrel protein in the outer mitochondrial membrane that is necessary for metabolite exchange with the cytosol and is proposed to be involved in certain forms of apoptosis. We studied the biogenesis of VDAC in human mitochondria by depleting the components of the mitochondrial import machinery by using RNA interference. Here, we show the importance of the translocase of the outer mitochondrial membrane (TOM) complex in the import of the VDAC precursor. The deletion of Sam50, the central component of the sorting and assembly machinery (SAM), led to both a strong defect in the assembly of VDAC and a reduction in the steady-state level of VDAC. Metaxin 2-depleted mitochondria had reduced levels of metaxin 1 and were deficient in import and assembly of VDAC and Tom40, but not of three matrix-targeted precursors. We also observed a reduction in the levels of metaxin 1 and metaxin 2 in Sam50-depleted mitochondria, implying a connection between these three proteins, although Sam50 and metaxins seemed to be in different complexes. We conclude that the pathway of VDAC biogenesis in human mitochondria involves the TOM complex, Sam50 and metaxins, and that it is evolutionarily conserved.
Figures




References
-
- Abdul KM, Terada K, Yano M, Ryan MT, Streimann I, Hoogenraad NJ, Mori M (2000) Functional analysis of human metaxin in mitochondrial protein import in cultured cells and its relationship with the Tom complex. Biochem Biophys Res Commun 276: 1028–1034 - PubMed
-
- Armstrong LC, Komiya T, Bergman BE, Mihara K, Bornstein P (1997) Metaxin is a component of a preprotein import complex in the outer membrane of the mammalian mitochondrion. J Biol Chem 272: 6510–6518 - PubMed
-
- Armstrong LC, Saenz AJ, Bornstein P (1999) Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J Cell Biochem 74: 11–22 - PubMed
-
- Blachly-Dyson E, Baldini A, Litt M, McCabe ERB, Forte M (1994) Human genes encoding the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane: mapping and identification of two new isoforms. Genomics 20: 62–67 - PubMed
-
- Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

