Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function
- PMID: 17510704
- PMCID: PMC1866249
- DOI: 10.1172/JCI30168
Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function
Abstract
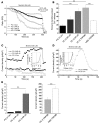
In recent years, the transient receptor potential melastatin member 8 (TRPM8) channel has emerged as a promising prognostic marker and putative therapeutic target in prostate cancer (PCa). However, the mechanisms of prostate-specific regulation and functional evolution of TRPM8 during PCa progression remain unclear. Here we show, for the first time to our knowledge, that only secretory mature differentiated human prostate primary epithelial (PrPE) luminal cells expressed functional plasma membrane TRPM8 ((PM)TRPM8) channels. Moreover, PCa epithelial cells obtained from in situ PCa were characterized by a significantly stronger (PM)TRPM8-mediated current than that in normal cells. This (PM)TRPM8 activity was abolished in dedifferentiated PrPE cells that had lost their luminal secretory phenotype. However, we found that in contrast to (PM)TRPM8, endoplasmic reticulum TRPM8 ((ER)TRPM8) retained its function as an ER Ca(2+) release channel, independent of cell differentiation. We hypothesize that the constitutive activity of (ER)TRPM8 may result from the expression of a truncated TRPM8 splice variant. Our study provides insight into the role of TRPM8 in PCa progression and suggests that TRPM8 is a potentially attractive target for therapeutic intervention: specific inhibition of either (ER)TRPM8 or (PM)TRPM8 may be useful, depending on the stage and androgen sensitivity of the targeted PCa.
Figures





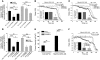

References
-
- Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Schalken J.A., van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11–20. - PubMed
-
- Hendriksen P.J., et al. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006;66:5012–5020. - PubMed
-
- Legrand G., et al. Ca2+ pools and cell growth. Evidence for sarcoendoplasmic Ca2+-ATPases 2B involvement in human prostate cancer cell growth control. J. Biol. Chem. 2001;276:47608–47614. - PubMed
-
- Thebault S., et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

