Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair
- PMID: 17510707
- PMCID: PMC1866253
- DOI: 10.1172/JCI30765
Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair
Erratum in
- J Clin Invest. 2008 Nov;118(11):3812
Abstract
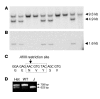
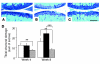
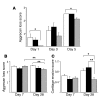
Aggrecan loss from cartilage in arthritis is mediated by aggrecanases. Aggrecanases cleave aggrecan preferentially in the chondroitin sulfate-2 (CS-2) domain and secondarily at the E(373) downward arrow(374)A bond in the interglobular domain (IGD). However, IGD cleavage may be more deleterious for cartilage biomechanics because it releases the entire CS-containing portion of aggrecan. Recent studies identifying aggrecanase-2 (ADAMTS-5) as the predominant aggrecanase in mouse cartilage have not distinguished aggrecanolysis in the IGD from aggrecanolysis in the CS-2 domain. We generated aggrecan knockin mice with a mutation that rendered only the IGD resistant to aggrecanases in order to assess the contribution of this specific cleavage to cartilage pathology. The knockin mice were viable and fertile. Aggrecanase cleavage in the aggrecan IGD was not detected in knockin mouse cartilage in situ nor following digestion with ADAMTS-5 or treatment of cartilage explant cultures with IL-1 alpha. Blocking cleavage in the IGD not only diminished aggrecan loss and cartilage erosion in surgically induced osteoarthritis and a model of inflammatory arthritis, but appeared to stimulate cartilage repair following acute inflammation. We conclude that blocking aggrecanolysis in the aggrecan IGD alone protects against cartilage erosion and may potentiate cartilage repair.
Figures




References
-
- Sandy J.D., Neame P.J., Boynton R.E., Flannery C.R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J. Biol. Chem. 1991;266:8683–8685. - PubMed
-
- Ilic M.Z., Handley C.J., Robinson H.C., Mok M.T. Mechanism of catabolism of aggrecan by articular cartilage. Arch. Biochem. Biophys. 1992;294:115–122. - PubMed
-
- Sandy J.D., Flannery C.R., Neame P.J., Lohmander L.S. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J. Clin. Invest. 1992;89:1512–1516. - PMC - PubMed
-
- Lohmander L.S., Neame P.J., Sandy J.D. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–1222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

