Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance
- PMID: 17510710
- PMCID: PMC1866250
- DOI: 10.1172/JCI30565
Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance
Abstract
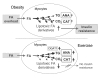
Increased fat deposition in skeletal muscle is associated with insulin resistance. However, exercise increases both intramyocellular fat stores and insulin sensitivity, a phenomenon referred to as "the athlete's paradox". In this study, we provide evidence that augmenting triglyceride synthesis in skeletal muscle is intrinsically connected with increased insulin sensitivity. Exercise increased diacylglycerol (DAG) acyltransferase (DGAT) activity in skeletal muscle. Channeling fatty acid substrates into TG resulted in decreased DAG and ceramide levels. Transgenic overexpression of DGAT1 in mouse skeletal muscle replicated these findings and protected mice against high-fat diet-induced insulin resistance. Moreover, in isolated muscle, DGAT1 deficiency exacerbated insulin resistance caused by fatty acids, whereas DGAT1 overexpression mitigated the detrimental effect of fatty acids. The heightened insulin sensitivity in the transgenic mice was associated with attenuated fat-induced activation of DAG-responsive PKCs and the stress mediator JNK1. Consistent with these changes, serine phosphorylation of insulin receptor substrate 1 was reduced, and Akt activation and glucose 4 membrane translocation were increased. In conclusion, upregulation of DGAT1 in skeletal muscle is sufficient to recreate the athlete's paradox and illustrates a mechanism of exercise-induced enhancement of muscle insulin sensitivity. Thus, increasing muscle DGAT activity may offer a new approach to prevent and treat insulin resistance and type 2 diabetes mellitus.
Figures
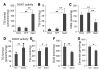
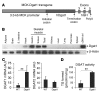

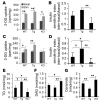

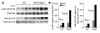
Comment in
- J Clin Invest. 117:1690.
References
-
- Unger R.H. Lipotoxic diseases. Annu. Rev. Med. 2002;53:319–336. - PubMed
-
- Yu Y.H., Ginsberg H.N. The role of acyl-CoA:diacylglycerol acyltransferase (DGAT) in energy metabolism. Ann. Med. 2004;36:252–261. - PubMed
-
- Phillips D.I., et al. Metabolism. Vol. 45. 1996. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. pp. 947–950. - PubMed
-
- Ebeling P., et al. Intramuscular triglyceride content is increased in IDDM. Diabetologia. 1998;41:111–115. - PubMed
-
- Manco M., et al. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism. . 2000;49:220–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

