Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups
- PMID: 17511499
- PMCID: PMC2430505
- DOI: 10.1021/bm0701088
Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups
Abstract
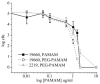
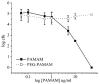
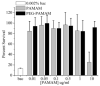
Poly(amidoamine) (PAMAM) dendrimer derivatives have been investigated for their biological applications, especially for delivery of drugs, including antimicrobial drugs to eukaryotic cells, but their effects on bacterial cells are largely unexplored. Herein we report that amino-terminated PAMAM dendrimers are highly toxic to the common Gram-negative pathogen Pseudomonas aeruginosa. The concentration that kills 50% of the bacteria (EC50) was in the range of approximately 0.9-1.5 microg/mL for the generation 5, amino-terminated dendrimers with or without partial (43%) coating of poly(ethylene glycol) (PEG). These EC50 values were lower than that ( approximately 1.9-2.8 microg/mL) for LL-37, a potent antimicrobial peptide expressed in a variety of epithelia. On the contrary, the dendrimers were far less toxic (EC50 > 21 microg/mL) to the Gram-positive pathogen Staphylococcus aureus than LL-37 (EC50 = approximately 1.9 microg/mL). In agreement with the previous studies on other cell types, the dendrimers were not cytotoxic to human corneal epithelial cells at the concentrations that were toxic to P. aeruginosa. Our findings indicate that amino-terminated PAMAM dendrimers and their partially PEG-coated derivatives possess attractive antimicrobial properties, particularly against Gram-negative bacteria, thus expanding the potential biological application of the dendrimers.
Figures


References
-
- Chen CZS, Cooper SL. Adv Mater. 2000;12:843–846.
-
- Abd-Elzaher MM, Ali IAI. Appl Organomet Chem. 2006;20:107–111.
-
- De Queiroz AAA, Abraham GA, Camillo MAP, Higa OZ, Silva GS, Fernandez MD, San Roman J. J Biomater Sci, Polym Ed. 2006;17:689–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

