The role of survivin in angiogenesis during zebrafish embryonic development
- PMID: 17511868
- PMCID: PMC1884147
- DOI: 10.1186/1471-213X-7-50
The role of survivin in angiogenesis during zebrafish embryonic development
Abstract
Background: Survivin is the smallest member of the inhibitor of apoptosis (IAP) gene family. Recently, the zebrafish survivin-1 gene has been cloned, showing remarkable sequence identity and similarity over the BIR domain compared with human and mouse survivin gene. Here we investigated the role of survivin in angiogenesis during zebrafish development. Morpholinos (MOs) targeting the 5' untranslated region (UTR) (SurUTR) and sequences flanking the initiation codon (SurATG) of zebrafish survivin-1 gene were injected into embryos at 1-4 cell stage. Vasculature was examined by microangiography and GFP expression in Tg(fli1:EGFP)y1 embryos.
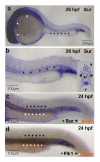
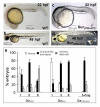
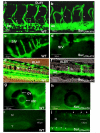
Results: In embryos co-injected with SurUTR and SurATG-MOs, vasculogenesis was intact but angiogenesis was markedly perturbed, especially in the inter-segmental vessels (ISV) and dorsal longitudinal anastomotic vessels (DLAV) of the trunk, the inner optic circle and optic veins of developing eyes and the sub-intestinal vessels. Apoptosis was increased, as shown by TUNEL staining and increase in caspase-3 activity. Efficacy of SurUTR and SurATG-MOs was demonstrated by translation inhibition of co-injected 5'UTR survivin:GFP plasmids. The phenotypes could be recapitulated by splice-site MO targeting the exon2-intron junction of survivin gene and rescued by survivin mRNA. Injection of human vascular endothelial growth factor (VEGF) protein induced ectopic angiogenesis and increased survivin expression, whereas treatment with a VEGF receptor inhibitor markedly reduced angiogenesis and suppressed survivin expression.
Conclusion: Survivin is involved in angiogenesis during zebrafish development and may be under VEGF regulation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

