Indole is an inter-species biofilm signal mediated by SdiA
- PMID: 17511876
- PMCID: PMC1899176
- DOI: 10.1186/1471-2180-7-42
Indole is an inter-species biofilm signal mediated by SdiA
Abstract
Background: As a stationary phase signal, indole is secreted in large quantities into rich medium by Escherichia coli and has been shown to control several genes (e.g., astD, tnaB, gabT), multi-drug exporters, and the pathogenicity island of E. coli; however, its impact on biofilm formation has not been well-studied.
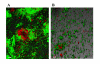
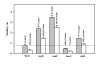
Results: Through a series of global transcriptome analyses, confocal microscopy, isogenic mutants, and dual-species biofilms, we show here that indole is a non-toxic signal that controls E. coli biofilms by repressing motility, inducing the sensor of the quorum sensing signal autoinducer-1 (SdiA), and influencing acid resistance (e.g., hdeABD, gadABCEX). Isogenic mutants showed these associated proteins are directly related to biofilm formation (e.g., the sdiA mutation increased biofilm formation 50-fold), and SdiA-mediated transcription was shown to be influenced by indole. The reduction in motility due to indole addition results in the biofilm architecture changing from scattered towers to flat colonies. Additionally, there are 12-fold more E. coli cells in dual-species biofilms grown in the presence of Pseudomonas cells engineered to express toluene o-monooxygenase (TOM, which converts indole to an insoluble indigoid) than in biofilms with pseudomonads that do not express TOM due to a 22-fold reduction in extracellular indole. Also, indole stimulates biofilm formation in pseudomonads. Further evidence that the indole effects are mediated by SdiA and homoserine lactone quorum sensing is that the addition of N-butyryl-, N-hexanoyl-, and N-octanoyl-L-homoserine lactones repress E. coli biofilm formation in the wild-type strain but not with the sdiA mutant.
Conclusion: Indole is an interspecies signal that decreases E. coli biofilms through SdiA and increases those of pseudomonads. Indole may be manipulated to control biofilm formation by oxygenases of bacteria that do not synthesize it in a dual-species biofilm. Furthermore, E. coli changes its biofilm in response to signals it cannot synthesize (homoserine lactones), and pseudomonads respond to signals they do not synthesize (indole).
Figures





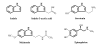
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

