Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex
- PMID: 17511968
- PMCID: PMC2910402
- DOI: 10.1016/j.biopsych.2006.12.003
Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex
Abstract
Background: Several lines of evidence suggest that N-methyl-D-aspartate (NMDA) receptor hypofunction may be associated with schizophrenia. Activation of metabotropic glutamate 5 (mGlu5) receptors enhances NMDA receptor mediated currents in vitro, implying that allosteric modulation of mGlu5 receptors may have therapeutic efficacy for schizophrenia. The aim of this study was to determine if positive allosteric modulators of mGlu5 receptors are effective in reversing two cellular effects of NMDA receptor antagonists that are relevant to schizophrenia: increases in corticolimbic dopamine neurotransmission and disruption of neuronal activity in the prefrontal cortex (PFC).
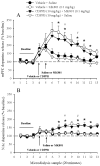
Methods: In freely moving rats, we measured the effects of the positive modulator of mGlu5 receptor 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) alone or in combination with the NMDA antagonist MK801 on 1) spontaneous firing and bursting of medial PFC (mPFC) neurons, and 2) dopamine release as measured by microdialysis in the mPFC and nucleus accumbens (NAc).
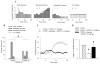
Results: The predominant effect of CDPPB on mPFC neurons was excitatory, leading to an overall excitatory population response. Pretreatment with CDPPB prevented MK801-induced excessive firing and reduced spontaneous bursting. In contrast, CDPPB had no significant effect on basal dopamine release as compared with control rats and did not alter MK801-induced activation of dopamine release in the mPFC and NAc.
Conclusions: These results show that positive modulation of mGlu5 receptors reverses the effects of noncompetitive NMDA antagonists on cortical neuronal firing without affecting dopamine neurotransmission. Thus, these compounds may be effective in ameliorating PFC mediated behavioral abnormalities that results from NMDA receptor hypofunction.
Figures


References
-
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, et al. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005;182:375–383. - PubMed
-
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MS. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. - PubMed
-
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. - PubMed
-
- Alagarsamy S, Rouse ST, Junge C, Hubert GW, Gutman D, Smith Y, et al. NMDA-induced phosphorylation and regulation of mGluR5. Pharmacol Biochem Behav. 2002;73:299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

