Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model
- PMID: 17512930
- PMCID: PMC1989132
- DOI: 10.1016/j.yexcr.2007.04.021
Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model
Abstract
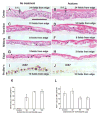
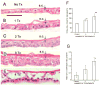
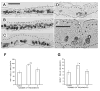
Repeated injury to the stratum corneum of mammalian skin (caused by friction, soaps, or organic solvents) elicits hyperkeratosis and epidermal thickening. Functionally, these changes serve to restore the cutaneous barrier and protect the organism. To better understand the molecular and cellular basis of this response, we have engineered an in vitro model of acetone-induced injury using organotypic epidermal cultures. Rat epidermal keratinocytes (REKs), grown on a collagen raft in the absence of any feeder fibroblasts, developed all the hallmarks of a true epidermis including a well-formed cornified layer. To induce barrier injury, REK cultures were treated with intermittent 30-s exposures to acetone then were fixed and paraffin-sectioned. After two exposures, increased proliferation (Ki67 and BrdU staining) was observed in basal and suprabasal layers. After three exposures, proliferation became confined to localized buds in the basal layer and increased terminal differentiation was observed (compact hyperkeratosis of the stratum corneum, elevated levels of K10 and filaggrin, and heightened transglutaminase activity). Thus, barrier disruption causes epidermal hyperplasia and/or enhances differentiation, depending upon the extent and duration of injury. Given that no fibroblasts are present in the model, the ability to mount a hyperplastic response to barrier injury is an inherent property of keratinocytes.
Figures




References
-
- Lampe MA, Williams ML, Elias PM. Human epidermal lipids: characterization and modulations during differentiation. J Lipid Res. 1983;24:131–140. - PubMed
-
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–126. - PubMed
-
- Proksch E, Holleran WM, Menon GK, Elias PM, Feingold KR. Barrier function regulates epidermal lipid and DNA synthesis. Br J Dermatol. 1993;128:473–482. - PubMed
-
- Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367–372. - PubMed
-
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

