Novel RDH12 mutations associated with Leber congenital amaurosis and cone-rod dystrophy: biochemical and clinical evaluations
- PMID: 17512964
- PMCID: PMC2441904
- DOI: 10.1016/j.visres.2007.04.005
Novel RDH12 mutations associated with Leber congenital amaurosis and cone-rod dystrophy: biochemical and clinical evaluations
Abstract
The purpose of this study was to determine the role of the retinol dehydrogenase 12 (RDH12) gene in patients affected with Leber congenital amaurosis (LCA), autosomal recessive retinitis pigmentosa (arRP) and autosomal dominant/recessive cone-rod dystrophies (CORD). Changes in the promoter region, coding regions and exon/intron junctions of the RDH12 gene were evaluated using direct DNA sequencing of patients affected with LCA (n=36 cases), RP (n=62) and CORD (n=21). The allele frequency of changes observed was assessed in a multiethnic control population (n=159 individuals). Detailed biochemical and structural modeling analysis of the observed mutations were performed to assess their biological role in the inactivation of Rdh12. A comprehensive clinical assessment of retinal structure and function in LCA patients carrying mutations in the RDH12 gene was completed. Of the six changes identified, three were novel including a homozygous C201R change in a patient affected with LCA, a heterozygous A177V change in patients affected with CORD and a heterozygous G46G change in a patient affected with LCA. A novel compound heterozygote T49M/A269fsX270 mutation was also found in a patient with LCA, and both homozygous and heterozygous R161Q changes were seen in 26 patients affected with LCA, CORD or RP. These R161Q, G46G and the A177V sequence changes were shown to be polymorphic. We found that Rdh12 mutant proteins associated with LCA were inactive or displayed only residual activity when expressed in COS-7 and Sf9 cells, whereas those mutants that were considered polymorphisms were fully active. Thus, impairment of retinal structure and function for patients carrying these mutations correlated with the biochemical properties of the mutants.
Figures
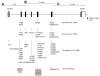

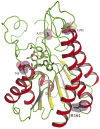
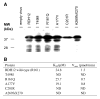



References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nature Genetics. 2001;28(1):92–95. - PubMed
-
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, et al. Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with leber congenital amaurosis. Investigative Ophthalmology and Visual Science. 2004;45(4):1259–1271. - PubMed
-
- Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): Catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44(18):7035–7047. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

