Are petals sterile stamens or bracts? The origin and evolution of petals in the core eudicots
- PMID: 17513305
- PMCID: PMC2533615
- DOI: 10.1093/aob/mcm076
Are petals sterile stamens or bracts? The origin and evolution of petals in the core eudicots
Abstract
Background: The aim of this paper is to discuss the controversial origins of petals from tepals or stamens and the links between the morphological expression of petals and floral organ identity genes in the core eudicots.
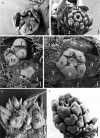
Scope: I challenge the widely held classical view that petals are morphologically derived from stamens in the core eudicots, and sepals from tepals or bracts. Morphological data suggest that tepal-derived petals have evolved independently in the major lineages of the core eudicots (i.e. asterids, Santalales and rosids) from Berberidopsis-like prototypes, and that staminodial petals have arisen only in few isolated cases where petals had been previously lost (Caryophyllales, Rosales). The clear correlation between continuous changes in petal morphology, and a scenario that indicates numerous duplications to have taken place in genes controlling floral organ development, can only be fully understood within a phylogenetic context. B-gene expression plays a fundamental role in the evolution of the petals by controlling petaloidy, but it does not clarify petal homology.
Conclusions: An increased synorganization of the flower in the core eudicots linked with the establishment of floral whorls restricts the petaloid gene expression to the second whorl, reducing the similarities of petals with tepals from which they were originally derived. An increased flower size linked with secondary polyandry or polycarpelly may lead to a breakdown of the restricted gene expression and a reversal to ancestral characteristics of perianth development. An altered 'sliding boundary' hypothesis is proposed for the core eudicots to explain shifts in petaloidy of the perianth and the event of staminodial petals. The repetitive changes of function in the perianth of the core eudicots are linked with shifts in petaloidy to the outer perianth whorl, or losses of petal or sepal whorls that can be secondarily compensated for by the inclusion of bracts in the flower. The origin and evolution of petals appears to be as complex on a molecular basis as it is from a morphological point of view.
Figures



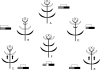
References
-
- Albert VA, Gustaffson MGH, Di Laurenzio L. Ontogenetic systematics, molecular developmental genetics, and the angiosperm petal. In: Soltis DE, Soltis PS, Doyle JJ, editors. Molecular systematics of plants II. New York: Kluwer Academic; 1998. pp. 349–374.
-
- Angiosperm Phylogeny Group (APG II) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society. 2003;141:399–436.
-
- von Balthazar M, Endress PK. Development of inflorescences and flowers in Buxaceae and the problem of perianth interpretation. International Journal of Plant Sciences. 2002;163:847–876.
-
- von Balthazar M, Schatz GE, Endress PK. Female flowers and inflorescences of Didymelaceae. Plant Systematics and Evolution. 2003;237:199–208.
-
- Bernhard A, Endress PK. Androecial development and systematics in Flacourtiaceae s.l. Plant Systematics and Evolution. 1999;215:141–155.

