Electrostatic and steric interactions determine bacteriorhodopsin single-molecule biomechanics
- PMID: 17513362
- PMCID: PMC1959538
- DOI: 10.1529/biophysj.106.101469
Electrostatic and steric interactions determine bacteriorhodopsin single-molecule biomechanics
Abstract
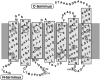
Bacteriorhodopsin (bR) is a haloarchaeal membrane protein that converts the energy of single photons into large structural changes to directionally pump protons across purple membrane. This is achieved by a complex combination of local dynamic interactions controlling bR biomechanics at the submolecular level, producing efficient amplification of the retinal photoisomerization. Using single molecule force spectroscopy at different salt concentrations, we show that tryptophan (Trp) residues use steric specific interactions to create a rigid scaffold in bR extracellular region and are responsible for the main unfolding barriers. This scaffold, which encloses the retinal, controls bR local mechanical properties and anchors the protein into the membrane. Furthermore, the stable Trp-based network allows ion binding to two specific sites on the extracellular loops (BC and FG), which are involved in proton release and lateral transport. In contrast, the cytoplasmic side of bR is mainly governed by relatively weak nonspecific electrostatic interactions that provide the flexibility necessary for large cytoplasmic structural rearrangements during the photocycle. The presence of an extracellular Trp-based network tightly enclosing the retinal seems common to most haloarchaeal rhodopsins, and could be relevant to their exceptional efficiency.
Figures





References
-
- Rief, M., M. Gautel, F. Oesterhelt, J. M. Fernandez, and H. E. Gaub. 1997. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 276:1109–1112. - PubMed
-
- Oberhauser, A. F., P. E. Marszalek, H. P. Erickson, and J. M. Fernandez. 1998. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 393:181–185. - PubMed
-
- Oesterhelt, F., D. Oesterhelt, M. Pfeiffer, A. Engel, H. E. Gaub, and D. J. Müller. 2000. Unfolding pathways of individual bacteriorhodopsins. Science. 288:143–146. - PubMed
-
- Kedrov, A., C. Ziegler, H. Janovjak, W. Kuhlbrandt, and D. J. Müller. 2004. Controlled unfolding and refolding of a single sodium-proton antiporter using atomic force microscopy. J. Mol. Biol. 340:1143–1152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

