The chloroplast Tat pathway utilizes the transmembrane electric potential as an energy source
- PMID: 17513364
- PMCID: PMC1959559
- DOI: 10.1529/biophysj.106.098731
The chloroplast Tat pathway utilizes the transmembrane electric potential as an energy source
Abstract
The thylakoid membrane, located inside the chloroplast, requires proteins transported across it for plastid biogenesis and functional photosynthetic electron transport. The chloroplast Tat translocator found on thylakoids transports proteins from the plastid stroma to the thylakoid lumen. Previous studies have shown that the chloroplast Tat pathway is independent of NTP hydrolysis as an energy source and instead depends on the thylakoid transmembrane proton gradient to power protein translocation. Because of its localization on the same membrane as the proton motive force-dependent F(0)F(1) ATPase, we believed that the chloroplast Tat pathway also made use of the thylakoid electric potential for transporting substrates. By adjusting the rate of photosynthetic proton pumping and by utilizing ionophores, we show that the chloroplast Tat pathway can also utilize the transmembrane electric potential for protein transport. Our findings indicate that the chloroplast Tat pathway is likely dependent on the total protonmotive force (PMF) as an energy source. As a protonmotive-dependent device, certain predictions can be made about structural features expected to be found in the Tat translocon, specifically, the presence of a proton well, a device in the membrane that converts electrical potential into chemical potential.
Figures
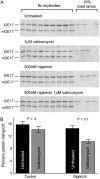

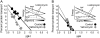

References
-
- Berks, B. C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393–404. - PubMed
-
- Cline, K., W. F. Ettinger, and S. M. Theg. 1992. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J. Biol. Chem. 267:2688–2696. - PubMed
-
- Mould, R., and C. Robinson. 1991. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J. Biol. Chem. 266:12189–12193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

