Structure and plasticity of the human immunodeficiency virus gp41 fusion domain in lipid micelles and bilayers
- PMID: 17513369
- PMCID: PMC1913135
- DOI: 10.1529/biophysj.106.102335
Structure and plasticity of the human immunodeficiency virus gp41 fusion domain in lipid micelles and bilayers
Abstract
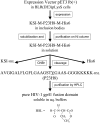
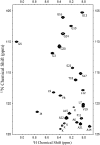
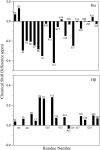
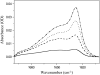
A thorough understanding of the structure of fusion domains of enveloped viruses in changing lipid environments helps us to formulate mechanistic models on how they might function in mediating viral entry by membrane fusion. We have expressed the N-terminal fusion domain of HIV-1 gp41 as a construct that is water-soluble in the absence of membranes, but that also binds with high affinity to lipid micelles and bilayers in their presence. We have solved the structure and studied the dynamics of this domain bound to dodecylphosphocholine micelles by homo- and heteronuclear NMR spectroscopy. The fusion peptide forms a stable hydrophobic helix from Ile(4) to Ala(14), but is increasingly more disordered and dynamic in a segment of intermediate polarity that stretches from Ala(15) to Ser(23). When bound to lipid bilayers at low concentration, the HIV fusion domain is also largely alpha-helical, as determined by CD and FTIR spectroscopy. However, at higher protein/lipid ratios, the domain is partially converted to form beta-structures in lipid bilayers. Controlled lipid mixing occurs at concentrations that support the alpha-helical, but not the beta-strand conformation.
Figures





Similar articles
-
Fusion activity of HIV gp41 fusion domain is related to its secondary structure and depth of membrane insertion in a cholesterol-dependent fashion.J Mol Biol. 2012 Apr 20;418(1-2):3-15. doi: 10.1016/j.jmb.2012.02.010. Epub 2012 Feb 17. J Mol Biol. 2012. PMID: 22343048 Free PMC article.
-
Conformational mapping of the N-terminal peptide of HIV-1 gp41 in membrane environments using (13)C-enhanced Fourier transform infrared spectroscopy.Biochim Biophys Acta. 2002 Feb 15;1559(2):96-120. doi: 10.1016/s0005-2736(01)00443-6. Biochim Biophys Acta. 2002. PMID: 11853678
-
Fully hydrophobic HIV gp41 adopts a hemifusion-like conformation in phospholipid bilayers.J Biol Chem. 2019 Oct 4;294(40):14732-14744. doi: 10.1074/jbc.RA119.009542. Epub 2019 Aug 13. J Biol Chem. 2019. PMID: 31409642 Free PMC article.
-
Combined NMR and EPR spectroscopy to determine structures of viral fusion domains in membranes.Biochim Biophys Acta. 2007 Dec;1768(12):3052-60. doi: 10.1016/j.bbamem.2007.09.010. Epub 2007 Sep 25. Biochim Biophys Acta. 2007. PMID: 17963720 Free PMC article. Review.
-
Functional domains within fusion proteins: prospectives for development of peptide inhibitors of viral cell fusion.Biosci Rep. 2000 Dec;20(6):535-55. doi: 10.1023/a:1010411021326. Biosci Rep. 2000. PMID: 11426693 Review.
Cited by
-
Recognition of membrane-bound fusion-peptide/MPER complexes by the HIV-1 neutralizing 2F5 antibody: implications for anti-2F5 immunogenicity.PLoS One. 2012;7(12):e52740. doi: 10.1371/journal.pone.0052740. Epub 2012 Dec 21. PLoS One. 2012. PMID: 23285173 Free PMC article.
-
Membrane protein structure and dynamics from NMR spectroscopy.Annu Rev Phys Chem. 2012;63:1-24. doi: 10.1146/annurev-physchem-032511-143731. Epub 2011 Nov 28. Annu Rev Phys Chem. 2012. PMID: 22136620 Free PMC article. Review.
-
Fusion Peptide of SARS-CoV-2 Spike Rearranges into a Wedge Inserted in Bilayered Micelles.J Am Chem Soc. 2021 Aug 25;143(33):13205-13211. doi: 10.1021/jacs.1c05435. Epub 2021 Aug 10. J Am Chem Soc. 2021. PMID: 34375093 Free PMC article.
-
Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme.Crit Rev Biochem Mol Biol. 2008 May-Jun;43(3):189-219. doi: 10.1080/10409230802058320. Crit Rev Biochem Mol Biol. 2008. PMID: 18568847 Free PMC article. Review.
-
Shallow boomerang-shaped influenza hemagglutinin G13A mutant structure promotes leaky membrane fusion.J Biol Chem. 2010 Nov 26;285(48):37467-75. doi: 10.1074/jbc.M110.153700. Epub 2010 Sep 8. J Biol Chem. 2010. PMID: 20826788 Free PMC article.
References
-
- Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 387:426–430. - PubMed
-
- Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777–810. - PubMed
-
- Gallaher, W. R. 1987. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 50:327–328. - PubMed
-
- Kliger, Y., and Y. Shai. 1997. A leucine zipper-like sequence from the cytoplasmic tail of the HIV-1 envelope glycoprotein binds and perturbs lipid bilayers. Biochemistry. 36:5157–5169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

