An overview of phenylalanine and tyrosine kinetics in humans
- PMID: 17513423
- PMCID: PMC2268015
- DOI: 10.1093/jn/137.6.1549S
An overview of phenylalanine and tyrosine kinetics in humans
Abstract
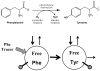
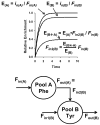
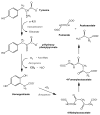
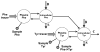
The initial use of a tracer of phenylalanine was by Moss and Schoenheimer in rats in 1940 to determine that phenylalanine was hydroxylated to tyrosine, defining for the first time the primacy of this pathway. Phenylalanine and tyrosine kinetics were not measured in humans until the 1970-80s. The first application was to determine the degree of blockage of phenylalanine hydroxylation in patients with hyperphenylalanemia and phenylketonuria, but this approach was expanded to determination of phenylalanine hydroxylation in normal subjects. Far more uses have been demonstrated for measuring rates of phenylalanine disposal and tyrosine production in relatively normal subjects than in patients with in-born errors of metabolism. Key to use of tracers to determine phenylalanine and tyrosine metabolic rates has been the development of appropriate tracer models. Most applications have used relatively simple models ignoring the intracellular hydroxylation rate component. Because the liver is the primary site of hydroxylation in the body, the intracellular enrichment at the site of hydroxylation can be assessed from the tracer enrichments at isotopic steady state in rapid-turnover plasma proteins, such as Apo-B, made and secreted by the liver. Although there are potential problems with use of deuterated tracers of phenylalanine, suitable tracers are available and have been demonstrated for general measurement of phenylalanine and tyrosine kinetics in humans.
Figures
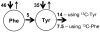
References
-
- Matthews DE. Proteins and amino acids. In: Shils ME, Shike M, Olson JA, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10. Philadelphia: Lippincott, Williams & Wilkins; 2006. pp. 23–60.
-
- Moss AR, Schoenheimer R. The conversion of phenylalanine to tyrosine in normal rats. J Biol Chem. 1940 Sep 1;135:415–29.
-
- Womack M, Rose WC. Feeding experiments with mixtures of highly purified amino acids. VI. the relation of phenylalanine and tyrosine to growth. J Biol Chem. 1934 Nov 1;107:449–58.
-
- O’Keefe SJD, Sender PM, James WPT. “Catabolic” loss of body nitrogen in response to surgery. Lancet. 1974;304:1035–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

