Two genes encoding protein kinases of the HstK family are involved in synthesis of the minor heterocyst-specific glycolipid in the cyanobacterium Anabaena sp. strain PCC 7120
- PMID: 17513480
- PMCID: PMC1951881
- DOI: 10.1128/JB.00323-07
Two genes encoding protein kinases of the HstK family are involved in synthesis of the minor heterocyst-specific glycolipid in the cyanobacterium Anabaena sp. strain PCC 7120
Abstract
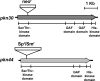
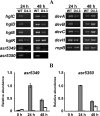
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 can fix N(2) under oxic conditions, and the activity of nitrogen fixation occurs exclusively in heterocysts, cells differentiated from vegetative cells in response to a limitation of a combined-nitrogen source in the growth medium. At the late stages of heterocyst differentiation, an envelope layer composed of two glycolipids is formed to limit the entry of oxygen so that the oxygen-sensitive nitrogenase can function. The genome of Anabaena sp. strain PCC 7120 possesses a family of 13 genes (the hstK family), all encoding proteins with a putative Ser/Thr kinase domain at their N termini and a His-kinase domain at their C termini. In this study, we showed that the double mutant D4.3 strain, in which two members of this gene family, pkn44 (all1625) and pkn30 (all3691), were both inactivated, failed to fix N(2) in the presence of oxygen (Fox(-)). In an environment without oxygen, a low level of nitrogenase activity was detectable (Fix(+)). Heterocyst development in the mutant D4.3 was delayed by 24 h and arrested at a relatively early stage without the formation of the glycolipid layer (Hgl(-)). Only the minor species of the two heterocyst-specific glycolipids (HGLs) was missing in the mutant. We propose that DevH, a putative transcription factor, coordinates the synthesis of both HGLs, while Pkn44/Pkn30 and the previously characterized PrpJ may represent two distinct regulatory pathways involved in the synthesis of the minor HGL and the major HGL, respectively.
Figures
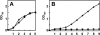



References
-
- Awai, K., and C. P. Wolk. 2007. Identification of the glycosyl transferase required for synthesis of the principal glycolipid characteristic of heterocysts of Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 266:98-102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

