The senescence-induced staygreen protein regulates chlorophyll degradation
- PMID: 17513504
- PMCID: PMC1913741
- DOI: 10.1105/tpc.106.044891
The senescence-induced staygreen protein regulates chlorophyll degradation
Abstract
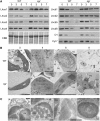
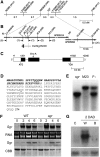
Loss of green color in leaves results from chlorophyll (Chl) degradation in chloroplasts, but little is known about how Chl catabolism is regulated throughout leaf development. Using the staygreen (sgr) mutant in rice (Oryza sativa), which maintains greenness during leaf senescence, we identified Sgr, a senescence-associated gene encoding a novel chloroplast protein. Transgenic rice overexpressing Sgr produces yellowish-brown leaves, and Arabidopsis thaliana pheophorbide a oxygenase-impaired mutants exhibiting a stay-green phenotype during dark-induced senescence have reduced expression of Sgr homologs, indicating that Sgr regulates Chl degradation at the transcriptional level. We show that the leaf stay-greenness of the sgr mutant is associated with a failure in the destabilization of the light-harvesting chlorophyll binding protein (LHCP) complexes of the thylakoid membranes, which is a prerequisite event for the degradation of Chls and LHCPs during senescence. Transient overexpression of Sgr in Nicotiana benthamiana and an in vivo pull-down assay show that Sgr interacts with LHCPII, indicating that the Sgr-LHCPII complexes are formed in the thylakoid membranes. Thus, we propose that in senescing leaves, Sgr regulates Chl degradation by inducing LHCPII disassembly through direct interaction, leading to the degradation of Chls and Chl-free LHCPII by catabolic enzymes and proteases, respectively.
Figures






References
-
- Akhtar, M.S., Goldschmidt, E., John, I., Rodoni, S., Matile, P., and Grierson, D. (1999). Altered patterns of senescence and ripening in gf, a stay-green mutant of tomato (Lycopersicon esculentum Mill.). J. Exp. Bot. 50 1115–1122.
-
- Armstead, I., et al. (2006). From crop to model to crop: Identifying the genetic basis of the staygreen mutation in the Lolium/Festuca forage and amenity grasses. New Phytol. 172 592–597. - PubMed
-
- Armstead, I., et al. (2007). Cross-species identification of Mendel's I locus. Science 315 73. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

