A randomised controlled trial to test the analgesic efficacy of topical morphine on minor superficial and partial thickness burns in accident and emergency departments
- PMID: 17513537
- PMCID: PMC2658275
- DOI: 10.1136/emj.2007.047324
A randomised controlled trial to test the analgesic efficacy of topical morphine on minor superficial and partial thickness burns in accident and emergency departments
Abstract
Objectives: To test the analgesic efficacy of topical morphine on superficial burns within the emergency department by comparing pain scores, comfort ratings and analgesia taken by participants.
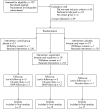
Method: A placebo-controlled three-treatment randomised controlled trial was undertaken. 59 participants were randomly allocated to receive a dressing containing Intrasite gel and morphine sulphate, Intrasite gel and water or the conventional Jelonet dressing. The study design enabled double-blinding between the two Intrasite gel treatments.
Results: 49 participants were included in the final analysis as 10 were lost to follow-up. No significant differences were observed between the pain scores or comfort ratings of the three treatments. Participants receiving Jelonet and the placebo reduced their pain scores by the greatest amount overall. However, participants receiving morphine were the only group to reduce pain scores by >20 mm on two consecutive time intervals (2 and 6 h). At 12 h the morphine group reported the highest pain scores. Only 4/15 participants receiving topical morphine administered additional analgesia compared with 12/17 receiving the Jelonet dressing and 6/17 receiving Intrasite and water (p = 0.055). However, when all analgesia was taken into account, the morphine group was administered the greatest amount. Overall, the placebo group reported their dressings to be the most comfortable and took the least amount of analgesia. Minor adverse reactions included itching, burning and a rash. No serious adverse reactions were reported.
Conclusions: Topical morphine sulphate does not seem to be as effective when used for the pain associated with superficial burns as when used for the pain associated with chronic inflammatory wounds. (The European Clinical Trials Database number for this study is 2005-003285-42.).
Conflict of interest statement
Competing interests: None.
References
-
- British Burn Association National Burn Care Review committee report—standards and strategy for burn care: a review of burn care in the British Isles. 2001. http://www.bapras.org.uk (accessed 2 Apr 2007)
-
- Department of Health Prodigy guidance: burns and scalds. 2004. http://www.prodigy.nhs.uk/guidance (Accessed 2 Apr 2007)
-
- Ptacek J T, Patterson D R, Doctor J. Describing and predicting the nature of procedural pain after thermal injuries: implications for research. J Burn Care Rehabil 200021318–326. - PubMed
-
- Geisser M E, Bingham H G, Robinson M E. Pain and anxiety during burn dressing changes: concordance between patients' and nurses' ratings and relation to medication administration and patient variables. J Burn Care Rehabil 199516165–171. - PubMed
-
- Alvi R, Jones S, Burrows D.et al The safety of topical anaesthetic and analgesic agents in a gel when used to provide pain relief at split skin donor sites. Burns 19982454–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical