Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light
- PMID: 17513597
- PMCID: PMC1932807
- DOI: 10.1128/AEM.02506-06
Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light
Abstract
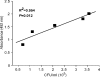
The fungus Cryptococcus neoformans possesses a polysaccharide capsule and can form biofilms on medical devices. We describe the characteristics of C. neoformans biofilm development using a microtiter plate model, microscopic examinations, and a colorimetric 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium-hydroxide (XTT) reduction assay to observe the metabolic activity of cryptococci within a biofilm. A strong correlation between XTT and CFU assays was demonstrated. Chemical analysis of the exopolymeric material revealed sugar composition consisting predominantly of xylose, mannose, and glucose, indicating the presence of other polysaccharides in addition to glucurunoxylomannan. Biofilm formation was affected by surface support differences, conditioning films on the surface, characteristics of the medium, and properties of the microbial cell. A specific antibody to the capsular polysaccharide of this fungus was used to stain the extracellular polysaccharide matrix of the fungal biofilms using light and confocal microscopy. Additionally, the susceptibility of C. neoformans biofilms and planktonic cells to environmental stress was investigated using XTT reduction and CFU assays. Biofilms were less susceptible to heat, cold, and UV light exposition than their planktonic counterparts. Our findings demonstrate that fungal biofilm formation is dependent on support surface characteristics and that growth in the biofilm state makes fungal cells less susceptible to potential environmental stresses.
Figures

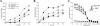
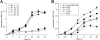
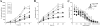
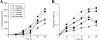
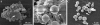


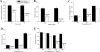
References
-
- Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, DC.
-
- Characklis, W. G., G. A. McFeters, and K. C. Marshall. 1990. Physiological ecology in biofilm systems, vol. 90. John Wiley & Sons, New York, NY.
-
- Fera, P., M. A. Siebel, W. G. Characklis, and D. Prieur. 1989. Seasonal variations in bacterial colonization of stainless steel, aluminum, and polycarbonate surfaces in seawater flow system. Biofouling 1:251-261.
-
- Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

