The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn
- PMID: 17513616
- PMCID: PMC1988937
- DOI: 10.1182/blood-2007-01-066092
The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn
Erratum in
- Blood. 2008 Mar 15;111(6):3299
Abstract
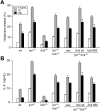
IgE/antigen-dependent mast cell activation plays a central role in immediate hypersensitivity and other allergic reactions. The Src family tyrosine kinase (SFK) Lyn is activated by the cross-linking of high-affinity IgE receptors (FcepsilonRI). Activated Lyn phosphorylates the FcepsilonRI subunits, beta and gamma, leading to subsequent activation of various signaling pathways. Lyn also plays a negative regulatory function by activating negative regulatory molecules. Another SFK, Fyn, also contributes to mast cell degranulation by inducing Gab2-dependent microtubule formation. Here we show that a third SFK, Hck, plays a critical role in mast cell activation. Degranulation and cytokine production are reduced in FcepsilonRI-stimulated hck(-/-) mast cells. The reduced degranulation can be accounted for by defects in Gab2 phosphorylation and microtubule formation. Importantly, Lyn activity is elevated in hck(-/-) cells, leading to increased phosphorylation of several negative regulators. However, positive regulatory events, such as activation of Syk, Btk, JNK, p38, Akt, and NF-kappaB, are substantially reduced in hck(-/-) mast cells. Analysis of lyn(-/-)hck(-/-), lyn(-/-)FcepsilonRIbeta(-/-), and hck(-/-)FcepsilonRIbeta(-/-) cells shows that Hck exerts these functions via both Lyn-dependent and Lyn-independent mechanisms. Thus, this study has revealed a hierarchical regulation among SFK members to fine-tune mast cell activation.
Figures





References
-
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. - PubMed
-
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–30. - PubMed
-
- Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. - PubMed
-
- Jouvin MH, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet JP. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem. 1994;269:5918–5925. - PubMed
-
- Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

