Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine
- PMID: 17513849
- PMCID: PMC1932884
- DOI: 10.1128/IAI.00076-07
Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine
Erratum in
- Infect Immun. 2009 Mar;77(3):1272
Abstract
Outer membrane proteins (OMPs) of microbial pathogens are critical components that mediate direct interactions between microbes and their surrounding environment. Consequently, the study of OMPs is integral to furthering the understanding of host-pathogen interactions and to identifying key targets for development of improved antimicrobial agents and vaccines. In this study, we used two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and tandem mass spectrometry to characterize the uropathogenic Escherichia coli (UPEC) outer membrane subproteome; 30 individual OMPs present on the bacterial surface during growth in human urine were identified. Fluorescence difference gel electrophoresis was used to identify quantitative changes in levels of UPEC strain CFT073 OMPs during growth in urine; six known receptors for iron compounds were induced in this environment, i.e., ChuA, IutA, FhuA, IroN, IreA, and Iha. A seventh putative iron compound receptor, encoded by CFT073 open reading frame (ORF) c2482, was also identified and found to be induced in urine. Further, the induction of these seven iron receptors in human urine and during defined iron limitation was verified by using quantitative real-time PCR (qPCR). An eighth iron receptor, fepA, displayed similar induction levels under these conditions as measured by qPCR but was not identified by 2D-PAGE. Addition of 10 microM FeCl(2) to human urine repressed the transcription of all eight iron receptor genes. A number of fecal-commensal, intestinal pathogenic, and uropathogenic E. coli strains all displayed similar growth rates in human urine, showing that the ability to grow in urine per se is not a urovirulence trait. Thus, human urine is an iron-limiting environment and UPEC enriches its outer membrane with iron receptors to contend with this iron limitation.
Figures
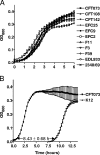

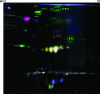



References
-
- Alban, A., S. O. David, L. Bjorkesten, C. Andersson, E. Sloge, S. Lewis, and I. Currie. 2003. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 336-44. - PubMed
-
- Asscher, A. W., M. Sussman, W. E. Waters, R. H. Davis, and S. Chick. 1966. Urine as a medium for bacterial growth. Lancet ii1037-1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

