Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia
- PMID: 17515402
- PMCID: PMC1988957
- DOI: 10.1182/blood-2006-10-055087
Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia
Abstract
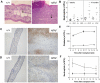
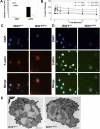
A pivotal mediator of actin dynamics is the protein cofilin, which promotes filament severing and depolymerization, facilitating the breakdown of existing filaments, and the enhancement of filament growth from newly created barbed ends. It does so in concert with actin interacting protein 1 (Aip1), which serves to accelerate cofilin's activity. While progress has been made in understanding its biochemical functions, the physiologic processes the cofilin/Aip1 complex regulates, particularly in higher organisms, are yet to be determined. We have generated an allelic series for WD40 repeat protein 1 (Wdr1), the mammalian homolog of Aip1, and report that reductions in Wdr1 function produce a dramatic phenotype gradient. While severe loss of function at the Wdr1 locus causes embryonic lethality, macrothrombocytopenia and autoinflammatory disease develop in mice carrying hypomorphic alleles. Macrothrombocytopenia is the result of megakaryocyte maturation defects, which lead to a failure of normal platelet shedding. Autoinflammatory disease, which is bone marrow-derived yet nonlymphoid in origin, is characterized by a massive infiltration of neutrophils into inflammatory lesions. Cytoskeletal responses are impaired in Wdr1 mutant neutrophils. These studies establish an essential requirement for Wdr1 in megakaryocytes and neutrophils, indicating that cofilin-mediated actin dynamics are critically important to the development and function of both cell types.
Figures



References
-
- Dawe HR, Minamide LS, Bamburg JR, Cramer LP. ADF/cofilin controls cell polarity during fibroblast migration. Curr Biol. 2003;13:252–257. - PubMed
-
- Chen J, Godt D, Gunsalus K, Kiss I, Goldberg M, Laski FA. Cofilin/ADF is required for cell motility during Drosophila ovary development and oogenesis. Nat Cell Biol. 2001;3:204–209. - PubMed
-
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. - PubMed
-
- Lappalainen P, Drubin DG. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

