Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial
- PMID: 17515584
- PMCID: PMC1867833
- DOI: 10.1503/cmaj.060837
Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial
Abstract
Background: Decision aids have been shown to be useful in selected situations to assist patients in making treatment decisions. Important features such as the format of decision aids and their graphic presentation of data on benefits and harms of treatment options have not been well studied.
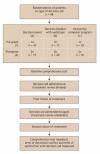
Methods: In a randomized trial with a 3 x 2 factorial design, we investigated the effects of decision aid format (decision board, decision booklet with audiotape, or interactive computer program) and graphic presentation of data (pie graph or pictogram) on patients' comprehension and choices of 3 treatments for anticoagulation, identified initially as "treatment A" (warfarin), "treatment B" (acetylsalicylic acid) and "treatment C" (no treatment). Patients aged 65 years or older without known atrial fibrillation and not currently taking warfarin were included. The effect of blinding to the treatment name was tested in a before-after comparison. The primary outcome was change in comprehension score, as assessed by the Atrial Fibrillation Information Questionnaire. Secondary outcomes were treatment choice, level of satisfaction with the decision aid, and decisional conflict.
Results: Of 102 eligible patients, 98 completed the study. Comprehension scores (maximum score 10) increased by an absolute mean of 3.1 (p < 0.01) after exposure to the decision aid regardless of the format or graphic presentation. Overall, 96% of the participants felt that the decision aid helped them make their treatment choice. Unblinding of the treatment name resulted in 36% of the participants changing their initial choice (p < 0.001).
Interpretation: The decision aid led to significant improvement in patients' knowledge regardless of the format or graphic representation of data. Revealing the name of the treatment options led to significant shifts in declared treatment preferences.
Figures
Comment in
-
Using decision aids to help patients navigate the "grey zone" of medical decision-making.CMAJ. 2007 May 22;176(11):1597-8. doi: 10.1503/cmaj.070490. CMAJ. 2007. PMID: 17515586 Free PMC article. No abstract available.
References
-
- Albers GW, Dalen JE, Laupacis A, et al. Antithrombotic therapy in atrial fibrillation. Chest 2001;119(1 Suppl):194S-206S. - PubMed
-
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449-57. - PubMed
-
- Stroke Prevention in Atrial Fibrillation Investigators. Preliminary report of the Stroke Prevention in Atrial Fibrillation Study. N Engl J Med 1990;322:863-8. - PubMed
-
- Peterson P, Gunrun B, Godtfredson J. Warfarin therapy and stroke prevention in patients with nonvaluvular atrial fibrillation. Geriatr Med Today 1990;8:61-73.
-
- Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1989;1:175-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical