The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses
- PMID: 17515603
- PMCID: PMC1952133
- DOI: 10.1128/MCB.02278-06
The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses
Abstract
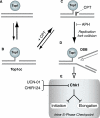
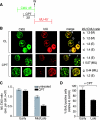
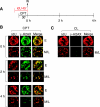
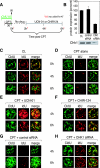
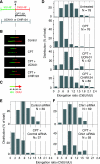
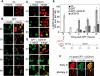
To investigate the contribution of DNA replication initiation and elongation to the intra-S-phase checkpoint, we examined cells treated with the specific topoisomerase I inhibitor camptothecin. Camptothecin is a potent anticancer agent producing well-characterized replication-mediated DNA double-strand breaks through the collision of replication forks with topoisomerase I cleavage complexes. After a short dose of camptothecin in human colon carcinoma HT29 cells, DNA replication was inhibited rapidly and did not recover for several hours following drug removal. That inhibition occurred preferentially in late-S-phase, compared to early-S-phase, cells and was due to both an inhibition of initiation and elongation, as determined by pulse-labeling nucleotide incorporation in replication foci and DNA fibers. DNA replication was actively inhibited by checkpoint activation since 7-hydroxystaurosporine (UCN-01), the specific Chk1 inhibitor CHIR-124, or transfection with small interfering RNA targeting Chk1 restored both initiation and elongation. Abrogation of the checkpoint markedly enhanced camptothecin-induced DNA damage at replication sites where histone gamma-H2AX colocalized with replication foci. Together, our study demonstrates that the intra-S-phase checkpoint is exerted by Chk1 not only upon replication initiation but also upon DNA elongation.
Figures


References
-
- Anglana, M., F. Apiou, A. Bensimon, and M. Debatisse. 2003. Dynamics of DNA replication in mammalian somatic cells: nucleotide pool modulates origin choice and interorigin spacing. Cell 114:385-394. - PubMed
-
- Berezney, R., D. D. Dubey, and J. A. Huberman. 2000. Heterogeneity of eukaryotic replicons, replicon clusters, and replication foci. Chromosoma 108:471-484. - PubMed
-
- Bjornsti, M. A., P. Benedetti, G. A. Viglianti, and J. C. Wang. 1989. Expression of human DNA topoisomerase I in yeast cells lacking yeast DNA topoisomerase I: restoration of sensitivity of the cells to the antitumor drug camptothecin. Cancer Res. 49:6318-6323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
