Epigenetic regulation of tumor necrosis factor alpha
- PMID: 17515611
- PMCID: PMC1951949
- DOI: 10.1128/MCB.02429-06
Epigenetic regulation of tumor necrosis factor alpha
Abstract
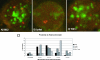
Tumor necrosis factor alpha (TNF-alpha) is a potent cytokine which regulates inflammation via the induction of adhesion molecules and chemokine expression. Its expression is known to be regulated in a complex manner with transcription, message turnover, message splicing, translation, and protein cleavage from the cell surface all being independently regulated. This study examined both cell lines and primary cells to understand the developmental regulation of epigenetic changes at the TNF-alpha locus. We demonstrate that epigenetic modifications of the TNF-alpha locus occur both developmentally and in response to acute stimulation and, importantly, that they actively regulate expression. DNA demethylates early in development, beginning with the hematopoietic stem cell. The TNF-alpha locus migrates from heterochromatin to euchromatin in a progressive fashion, reaching euchromatin slightly later in differentiation. Finally, histone modifications characteristic of a transcriptionally competent gene occur with myeloid differentiation and progress with differentiation. Additional histone modifications characteristic of active gene expression are acquired with stimulation. In each case, manipulation of these epigenetic variables altered the ability of the cell to express TNF-alpha. These studies demonstrate the importance of epigenetic regulation in the control of TNF-alpha expression. These findings may have relevance for inflammatory disorders in which TNF-alpha is overproduced.
Figures








References
-
- Amour, A., P. M. Slocombe, A. Webster, M. Butler, C. G. Knight, B. J. Smith, P. E. Stephens, C. Shelley, M. Hutton, V. Knauper, A. J. Docherty, and G. Murphy. 1998. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 435:39-44. - PubMed
-
- Armenante, F., M. Merola, A. Furia, and M. Palmieri. 1999. Repression of the IL-6 gene is associated with hypermethylation. Biochem. Biophys. Res. Commun. 258:644-647. - PubMed
-
- Askling, J., M. Fored, E. Baecklund, L. Brandt, C. Backlin, A. Ekbom, C. Sundstrom, L. Bertilsson, L. Coster, P. Geborek, L. Jacobsson, S. Lindblad, J. Lysholm, S. Rantapaa-Dahlqvist, T. Saxne, L. Klareskog, and N. Feltelius. 2005. Hematopoietic malignancies in rheumatoid arthritis. Lymphoma risk and characteristics following TNF-antagonists. Ann. Rheum. Dis. 64:1414-1420. (First published 20 April 2005; doi: 10.1136/ard.2004.033241.) - DOI - PMC - PubMed
-
- Barthel, R., and A. E. Goldfeld. 2003. T cell-specific expression of the human TNF-alpha gene involves a functional and highly conserved chromatin signature in intron 3. J. Immunol. 171:3612-3619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
