Patch-clamp 'mapping' of ion channel activity in human sperm reveals regionalisation and co-localisation into mixed clusters
- PMID: 17516540
- PMCID: PMC3549611
- DOI: 10.1002/jcp.21153
Patch-clamp 'mapping' of ion channel activity in human sperm reveals regionalisation and co-localisation into mixed clusters
Abstract
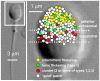
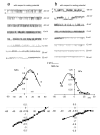
Ion channels are pivotal to many aspects of sperm physiology and function. We have used the patch clamp technique to investigate the distribution of ion channels in the plasma membrane of the head of human spermatozoa. We report that three types of activity are common in the equatorial and acrosomal regions of the sperm head. Two of these (a chloride-permeable anion channel showing long stable openings and a second channel which flickered between open and closed states and was dependent upon cytoplasmic factors for activity) were localised primarily to the equatorial segment. A third type, closely resembling the flickering activity but with different voltage sensitivity of P(open), was more widely distributed but was not detectable over the anterior acrosome. In the anterior acrosomal area channels were present but showed very low levels of spontaneous activity. A unique feature of channel activity in the sperm equatorial region was co-localisation into mixed clusters, most patches were devoid of activity but 'active' patches typically contained two or more types of activity (in a single 200-300 nM diameter patch). We conclude that ion channels in the sperm membrane show regionalisation of type and activity and that the channels are clustered into functional groups, possibly interacting through local effects on membrane potential.
2007 Wiley-Liss, Inc.
Figures




References
-
- Alavi SM, Cosson J. Sperm motility in fishes. I. Effects of temperature and pH: a review. Cell Biol Int. 2005;29:101–110. - PubMed
-
- Barfield JP, Yeung CH, Cooper TG. Characterization of potassium channels involved in volume regulation of human spermatozoa. Mol Hum Reprod. 2005;11:891–897. - PubMed
-
- Barratt CL, Publicover SJ. Interaction between sperm and zona pellucida in male fertility. Lancet. 2001;358:1660–1662. - PubMed
-
- Bedford JM, Moore HD, Franklin LE. Significance of the equatorial segment of the acrosome of the spermatozoon in eutherian mammals. Exp Cell Res. 1979;119:119–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

