syn-1,2-Amino alcohols via diastereoselective allylic C-H amination
- PMID: 17516648
- PMCID: PMC2720786
- DOI: 10.1021/ja071905g
syn-1,2-Amino alcohols via diastereoselective allylic C-H amination
Figures



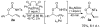
References
-
-
C–O to C–N catalyzed by Pd(0): Trost BM, Sudhakar AR. J Am Chem Soc. 1987;109:3792.Trost BM, Patterson DE. J Org Chem. 1998;63:1339.Hayashi T, Yamamoto A, Ito Y. Tetrahedron Lett. 1988;29:99.Bystrom SE, Aslanian R, Backvall JE. Tetrahedron Lett. 1985;26:1749.Hayashi T, Kishi K, Yamamoto A, Ito Y. Tetrahedron Lett. 1990;31:1743.Evans PA, Robinson JE, Nelson JD. J Am Chem Soc. 1999;121:6761.Ohmura T, Hartwig JF. J Am Chem Soc. 2002;124:15164.Olefin aminohydroxylation: Kolb HC, Sharpless KB. In: Transition Metals for Organic Synthesis. 2. Beller M, Bolm C, editors. Wiley-VCH Verlag; Weinheim, Germany: 2004. p. 309.Mannich: Trost BM, Terrell LR. J Am Chem Soc. 2003;125:338.Matsunaga S, Yoshida T, Morimoto H, Kumagai N, Shibasaki M. J Am Chem Soc. 2004;126:8777.Ramasastry SSV, Zhang H, Tanaka F, Barbas CF. J Am Chem Soc. 2007;129:288.
-
-
-
Streamlining syntheses via late stage C–H oxidation see: Fraunhoffer KJ, Bachovchin DA, White MC. Org Lett. 2005;7:223.Covell DJ, Vermeulen NA, Labenz NA, White MC. Angew Chem, Int Ed. 2006;45:8217.Hoffman RW. Synthesis. 2006;21:3531.Elegant examples of late stage C–H hydroxylation and amination see: Wender PA, Hilinski MK, Mayweg AVW. Org Lett. 2005;7:79.Hinman A, Du Bois J. J Am Chem Soc. 2003;125:11510.
-
-
-
C-H amination via metal nitrenes: Espino CG, Du Bois J. Angew Chem, Int Ed. 2001;40:598.Kim M, Mulcahy JV, Espino CG, Du Bois J. Org Lett. 2006;8:1073.Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198.
-
-
-
For single examples of catalytic allylic C–H amination: (a) ref 3b. Larock RC, Hightower TR, Hasvold LA, Peterson KP. J Org Chem. 1996;61:3584.
-
-
-
Hegedus LS, Allen GF, Waterman EL. J Am Chem Soc. 1976;98:2674.Ronn M, Backvall JE, Andersson PG. Tetrahedron Lett. 1995;36:7749. (c) ref. 4b. Overman LE, Remarchuk TP. J Am Chem Soc. 2002;124:12.Brice JL, Harang JE, Timokhin VI, Anastasi NR, Stahl SS. J Am Chem Soc. 2005;127:2868.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

