Minimal effect of MDR1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of indinavir in HIV-infected patients
- PMID: 17517050
- PMCID: PMC2000655
- DOI: 10.1111/j.1365-2125.2007.02903.x
Minimal effect of MDR1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of indinavir in HIV-infected patients
Abstract
Aims: The protease inhibitor indinavir is characterized by an important interindividual pharmacokinetic variability, which results from the actions of the metabolizing enzymes cytochrome P450 (CYP) 3A and the multidrug efflux pump P-glycoprotein (P-gp), encoded by MDR1. Using a population pharmacokinetic approach, we investigated the effect of several MDR1 and CYP3A5 polymorphisms on the pharmacokinetic parameters of indinavir in HIV-infected patients.
Methods: Twenty-eight patients receiving indinavir alone or together with ritonavir were included. Indinavir pharmacokinetics were studied over a 12 h interval. Genetic polymorphisms were assessed by real-time PCR assays and direct sequencing for MDR1 and by PCR-SSCP analysis for CYP3A5.
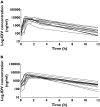
Results: The pharmacokinetics of indinavir were best described by a one-compartment model with first-order absorption. In the final model, the MDR1 C3435T genotype and ritonavir were identified as statistically significant covariates (P </= 0.001) for the absorption rate constant (95% confidence interval on the difference between CC and CT genotype 0.37, 5.53) and for clearance (95% confidence interval on the difference 5.8, 26.2), respectively. Patients with the CYP3A5*3/*3 genotype receiving indinavir alone had a 31% decrease in the indinavir clearance rate compared with patients carrying the CYP3A5*1/*3 genotype.
Conclusions: The MDR1 C3435T genotype affects the absorption constant of indinavir suggesting that P-gp may be implicated in its pharmacokinetic variability. Through its inhibition of CYP3A and P-gp, ritonavir could attenuate the pharmacokinetic variability linked to genetic differences, reducing significantly the interindividual variability of indinavir. However, genotyping MDR1 and/or CYP3A5 to optimize protease inhibitor boosted regimens does not seem clinically relevant.
Figures
References
-
- Molla A, Granneman GR, Sun E, Kempf DJ. Recent developments in HIV protease inhibitor therapy. Antiviral Res. 1998;39:1–23. - PubMed
-
- Slain D, Pakyz A, Israel DS, Monroe S, Polk RE. Variability in activity of hepatic CYP3A4 in patients infected with HIV. Pharmacotherapy. 2000;20:898–907. - PubMed
-
- Barry M, Gibbons S, Back D, Mulcahy F. Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet. 1997;32:194–209. - PubMed
-
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous