Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field
- PMID: 17517599
- PMCID: PMC1890523
- DOI: 10.1073/pnas.0701104104
Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field
Abstract
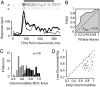
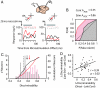
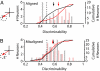
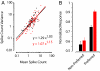
Visual attention provides a means of selecting among the barrage of information reaching the retina and of enhancing the perceptual discriminability of relevant stimuli. Neurophysiological studies in monkeys and functional imaging studies in humans have demonstrated neural correlates of these perceptual improvements in visual cortex during attention. Importantly, voluntary attention improves the discriminability of visual cortical responses to relevant stimuli. Recent work aimed at identifying sources of attentional modulation has implicated the frontal eye field (FEF) in driving spatial attention. Subthreshold microstimulation of the FEF enhances the responses of area V4 neurons to spatially corresponding stimuli. However, it is not known whether these enhancements include improved visual-response discriminability, a hallmark of voluntary attention. We used receiver-operator characteristic analysis to quantify how well V4 responses discriminated visual stimuli and examined how discriminability was affected by FEF microstimulation. Discriminability of responses to stable visual stimuli decayed over time but was transiently restored after microstimulation of the FEF. As observed during voluntary attention, the enhancement resulted only from changes in the magnitude of V4 responses and not in the relationship between response magnitude and variance. Enhanced response discriminability was apparent immediately after microstimulation and was reliable within 40 ms of microstimulation onset, indicating a direct influence of FEF stimulation on visual representations. These results contribute to the mounting evidence that saccade-related signals are a source of spatial attentive selection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Posner MI. Q J Exp Psychol. 1980;32:3–25. - PubMed
-
- Cameron EL, Tai JC, Carrasco M. Vision Res. 2002;42:949–967. - PubMed
-
- Carrasco M, Williams PE, Yeshurun Y. J Vis. 2002;2:467–479. - PubMed
-
- Lee DK, Koch C, Braun J. Vision Res. 1997;37:2409–2418. - PubMed
-
- Moran J, Desimone R. Science. 1985;229:782–784. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

