Protection of the glutamate pool concentration in enteric bacteria
- PMID: 17517610
- PMCID: PMC1890519
- DOI: 10.1073/pnas.0703360104
Protection of the glutamate pool concentration in enteric bacteria
Abstract
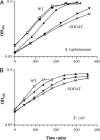
The central nitrogen metabolic circuit in enteric bacteria consists of three enzymes: glutamine synthetase, glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH). With the carbon skeleton provided by 2-oxoglutarate, ammonia/ammonium (NH(4)(+)) is assimilated into two central nitrogen intermediates, glutamate and glutamine. Although both serve as nitrogen donors for all biosynthetic needs, glutamate and glutamine play different roles. Internal glutamine serves as a sensor of external nitrogen availability, and its pool concentration decreases upon nitrogen limitation. A high glutamate pool concentration is required to maintain the internal K(+) pool. The configuration of high glutamate and low glutamine pools was disrupted in GOGAT(-) mutants under low NH(4)(+) conditions: the glutamate pool was low, the difference between glutamate and glutamine was diminished, and growth was defective. When a GOGAT(-) mutant was cultured in an NH(4)(+)-limited chemostat, two sequential spontaneous mutations occurred. Each resulted in a suppressor mutant that outgrew its predecessor in the chemostat. The first suppressor overexpressed GDH, and the second also had a partially impaired glutamine synthetase. The result was a triple mutant in which NH(4)(+) was assimilated by two enzymes instead of the normal three and yet glutamate and glutamine pools and growth were essentially normal. The results indicate preference for the usual ratio of glutamate and glutamine and the resilient and compensatory nature of the circuit on pool control. Analysis of other suppressor mutants selected on solid medium suggests that increased GDH expression is the key for rescue of the growth defect of GOGAT(-) mutants under low NH(4)(+) conditions.
Conflict of interest statement
The author declares no conflict of interest.
Figures




References
-
- Reitzer L. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Bock A, Curtiss R III, Kaper JB, Neidhardt FC, Nystrom T, Rudd KE, Squires CL, editors. Washington, DC: Am Soc Microbiol; 2004. [Accessed May 2, 2006]. Chap 3.6.1.3. Available at www.ecosal.org.
-
- Stadtman ER. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Bock A, Curtiss R III, Kaper JB, Neidhardt FC, Nystrom T, Rudd KE, Squires CL, editors. Vol. 30. Washington, DC: Am Soc Microbiol; 2006. [Accessed April 2004]. Chap 3.6.1.6. Available at www.ecosal.org.
-
- Miller RE, Stadtman ER. J Biol Chem. 1972;247:7407–7419. - PubMed
-
- Wohlheuter RM, Schutt H, Holzer H. In: The Enzymes of Glutamine Metabolism. Prusiner SB, Stadtman ER, editors. New York: Academic; 1973. pp. 45–64.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

