Nanoprobes with near-infrared persistent luminescence for in vivo imaging
- PMID: 17517614
- PMCID: PMC1890483
- DOI: 10.1073/pnas.0702427104
Nanoprobes with near-infrared persistent luminescence for in vivo imaging
Abstract
Fluorescence is increasingly used for in vivo imaging and has provided remarkable results. Yet this technique presents several limitations, especially due to tissue autofluorescence under external illumination and weak tissue penetration of low wavelength excitation light. We have developed an alternative optical imaging technique by using persistent luminescent nanoparticles suitable for small animal imaging. These nanoparticles can be excited before injection, and their in vivo distribution can be followed in real-time for more than 1 h without the need for any external illumination source. Chemical modification of the nanoparticles' surface led to lung or liver targeting or to long-lasting blood circulation. Tumor mass could also be identified on a mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


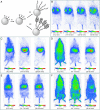
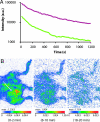
Comment in
-
Early Development of nanoprobes for diagnostic imaging.Liver Transpl. 2007 Nov;13(11):1604. Liver Transpl. 2007. PMID: 18064764
References
-
- Cherry SR. Annu Rev Biomed Eng. 2006;8:35–62. - PubMed
-
- Dubertret B, Skourides P, Noriis DJ, Noireaux V, Brivanlou AH, Libchader A. Science. 2002;298:1759–1762. - PubMed
-
- So MK, Xu C, Loening AM, Gambhir SS, Rao J. Nat Biotech. 2006;24:339–343. - PubMed
-
- Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Bioconjugate Chem. 2004;15:79–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

