Primary protein response after ligand photodissociation in carbonmonoxy myoglobin
- PMID: 17517618
- PMCID: PMC1887578
- DOI: 10.1073/pnas.0611560104
Primary protein response after ligand photodissociation in carbonmonoxy myoglobin
Abstract
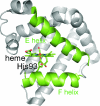
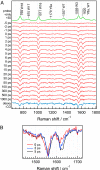
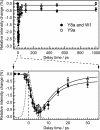
Time-resolved UV resonance Raman (UVRR) spectroscopic studies of WT and mutant myoglobin were performed to reveal the dynamics of protein motion after ligand dissociation. After dissociation of carbon monoxide (CO) from the heme, UVRR bands of Tyr showed a decrease in intensity with a time constant of 2 ps. The intensity decrease was followed by intensity recovery with a time constant of 8 ps. On the other hand, UVRR bands of Trp residues located in the A helix showed an intensity decrease that was completed within the instrument response time. The intensity decrease was followed by an intensity recovery with a time constant of approximately 50 ps and lasted up to 1 ns. The time-resolved UVRR study of the myoglobin mutants demonstrated that the hydrophobicity of environments around Trp-14 decreased, whereas that around Trp-7 barely changed in the primary protein response. The present data indicate that displacement of the E helix toward the heme occurs within the instrument response time and that movement of the FG corner takes place with a time constant of 2 ps. The finding that the instantaneous motion of the E helix strongly suggests a mechanism in which protein structural changes are propagated from the heme to the A helix through the E helix motion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Perutz MF, Fermi G, Luisi B, Shaanan B, Liddington RC. Acc Chem Res. 1987;20:309–321. - PubMed
-
- Dickerson RE, Geiss I. Hemoglobin: Structure, Function, Evolution, and Pathology. Menlo Park, CA: Benjamin Cummings; 1983.
-
- Jain R, Chan MK. J Biol Inorg Chem. 2003;8:1–11. - PubMed
-
- Kachalova GS, Popov AN, Bartunik HD. Science. 1999;284:473–476. - PubMed
-
- Goodno GD, Astinov V, Miller RJD. J Phys Chem A. 1999;103:10630–10643.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

