In vitro interactions between apricitabine and other deoxycytidine analogues
- PMID: 17517847
- PMCID: PMC1932514
- DOI: 10.1128/AAC.01204-06
In vitro interactions between apricitabine and other deoxycytidine analogues
Abstract
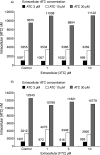
Apricitabine is a novel deoxycytidine analogue reverse transcriptase inhibitor that is under development for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. Apricitabine is phosphorylated to its active triphosphate by deoxycytidine kinase, which is also responsible for the intracellular phosphorylation of lamivudine (3TC) and emtricitabine (FTC); hence, in vitro studies were performed to investigate possible interactions between apricitabine and these agents. Human peripheral blood mononuclear cells (PBMC) were incubated for 24 h with various concentrations of (3)H-labeled or unlabeled apricitabine, 3TC, or FTC. Intracellular concentrations of parent compounds and their phosphorylated derivatives were measured by high-performance liquid chromatography. In other experiments, viral reverse transcriptase activity was measured in PBMC infected with HIV-1 bearing M184V in the presence of various concentrations of apricitabine and 3TC. [(3)H]apricitabine and [(3)H]3TC were metabolized intracellularly to form mono-, di-, and triphosphates. 3TC and FTC (1 to 10 microM) produced concentration-dependent decreases in apricitabine phosphorylation; in contrast, apricitabine at concentrations of up to 30 muM had no effect on the phosphorylation of 3TC or FTC. The combination of apricitabine and 3TC reduced the antiviral activity of apricitabine against HIV-1: apricitabine concentrations producing 50% inhibition of viral reverse transcriptase were increased two- to fivefold in the presence of 3TC. These findings suggest that nucleoside reverse transcriptase inhibitors with similar modes of action may show biochemical interactions that affect their antiviral efficacy. It is therefore essential that potential interactions between combinations of new and existing agents be thoroughly investigated before such combinations are introduced into clinical practice.
Figures
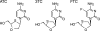


References
-
- Bethell, R. C., Y. S. Lie, and N. T. Parkin. 2005. In vitro activity of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor (NRTI), against 215 HIV-1 isolates resistant to other NRTIs. Antivir. Chem. Chemother. 16:295-302. - PubMed
-
- Blaise, P., P. Clevenbergh, D. Vaira, M. Moutschen, and P. Dellamonica. 2002. HIV resistance to antiretroviral drugs: mechanisms, genotypic and phenotypic resistance testing in clinical practice. Acta Clin. Belg. 57:191-201. - PubMed
-
- Cahn, P., I. Cassetti, R. Wood, P. Phanuphak, L. Shiveley, R. C. Bethell, and J. Sawyer. 2006. Efficacy and tolerability of 10-day monotherapy with apricitabine in antiretroviral-naive, HIV-infected patients. AIDS 20:1261-1268. - PubMed
-
- Cohen, C., C. Katlama, R. Murphy, J. Gathe, C. Brinson, G. Richmond, P. M. Girard, J. Fessel, A. Liappis, E. Puglia, B. Rodwick, J. Nadler, W. O'Brien, K. Arasteh, M. Otto, S. Viitanen-Erickson, and R. Levy. 2005. Antiretroviral activity and tolerability of Reverset (D-d4FC), a new fluoro-cytidine nucleoside analog, when used in combination therapy in treatment-experienced patients: results of phase IIb study RVT-203. Abstr. Third IAS, abstr. WeOaLB0103.
-
- Delehanty, J., C. Wakeford, L. Hulett, J. Quinn, B. Mccreedy, M. Almond, D. Miralles, and F. Rousseau. 1999. A phase I/II randomized, controlled study of FTC versus 3TC in HIV-infected patients. Abstr. Sixth CROI, abstr. 16.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

