Population pharmacokinetics of tafenoquine during malaria prophylaxis in healthy subjects
- PMID: 17517850
- PMCID: PMC1932489
- DOI: 10.1128/AAC.01183-06
Population pharmacokinetics of tafenoquine during malaria prophylaxis in healthy subjects
Abstract
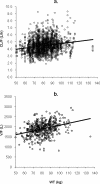
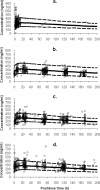
The population pharmacokinetics of tafenoquine were studied in Australian soldiers taking tafenoquine for malarial prophylaxis. The subjects (476 males and 14 females) received a loading dose of 200 mg tafenoquine base daily for 3 days, followed by a weekly dose of 200 mg tafenoquine for 6 months. Blood samples were collected from each subject after the last loading dose and then at weeks 4, 8, and 16. Plasma tafenoquine concentrations were determined by liquid chromatography-tandem mass spectrometry. Population modeling was performed with NONMEM, using a one-compartment model. Typical values of the first-order absorption rate constant (K(a)), clearance (CL/F), and volume of distribution (V/F) were 0.243 h(-1), 0.056 liters/h/kg, and 23.7 liters/kg, respectively. The intersubject variability (coefficient of variation) in CL/F and V/F was 18% and 22%, respectively. The interoccasion variability in CL/F was 18%, and the mean elimination half-life was 12.7 days. A positive linear association between weight and both CL/F and V/F was found, but this had insufficient impact to warrant dosage adjustments. Model robustness was assessed by a nonparametric bootstrap (200 samples). A degenerate visual predictive check indicated that the raw data mirrored the postdose concentration-time profiles simulated (n = 1,000) from the final model. Individual pharmacokinetic estimates for tafenoquine did not predict the prophylactic outcome with the drug for four subjects who relapsed with Plasmodium vivax malaria, as they had similar pharmacokinetics to those who were free of malaria infection. No obvious pattern existed between the plasma tafenoquine concentration and the pharmacokinetic parameter values for subjects with and without drug-associated moderate or severe adverse events. This validated population pharmacokinetic model satisfactorily describes the disposition and variability of tafenoquine used for long-term malaria prophylaxis in a large cohort of soldiers on military deployment.
Figures
References
-
- Brueckner, R. P., K. C. Lasseter, E. T. Lin, and B. G. Schuster. 1998. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. Am. J. Trop. Med. Hyg. 58:645-649. - PubMed
-
- Ette, E. I., and T. M. Ludden. 1995. Population pharmacokinetic modelling: the importance of informative graphics. Pharm. Res. 12:1845-1855. - PubMed
-
- Ette, E. I. 1997. Population modelling stability and performance. J. Clin. Pharmacol. 37:486-495. - PubMed
-
- Ette, E. I., P. J. Williams, and J. R. Lane. 2004. Population pharmacokinetics. III. Design, analysis, and application of population pharmacokinetic studies. Ann. Pharmacother. 38:2136-2144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

