Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament
- PMID: 17517862
- PMCID: PMC1951984
- DOI: 10.1128/IAI.00159-07
Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament
Abstract
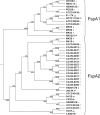
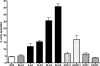
Cj0859c, or FspA, is a small, acidic protein of Campylobacter jejuni that is expressed by a sigma(28) promoter. Analysis of the fspA gene in 41 isolates of C. jejuni revealed two overall variants of the predicted protein, FspA1 and FspA2. Secretion of FspA occurs in broth-grown bacteria and requires a minimum flagellar structure. The addition of recombinant FspA2, but not FspA1, to INT407 cells in vitro resulted in a rapid induction of apoptosis. These data define a novel C. jejuni virulence factor, and the observed heterogeneity among fspA alleles suggests alternate virulence potential among different strains.
Figures




References
-
- Alm, R. A., L.-S. L. Ling, D. Moir, B. L. King, E. D. Brown, P. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. de Jonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jian, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:160-189. - PubMed
-
- Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. - PubMed
-
- Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

