Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice
- PMID: 17517868
- PMCID: PMC1951993
- DOI: 10.1128/IAI.01335-06
Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice
Abstract
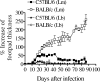
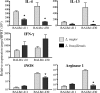
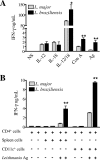
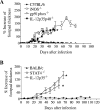
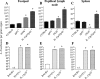
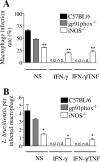
Cutaneous leishmaniasis is caused by protozoan parasites of the genus Leishmania. The mechanisms of pathogen control have been established primarily in the mouse model of Leishmania major infection, but they might not hold true for other Leishmania species associated with cutaneous disease. Here, we analyzed the role of cytokines, signaling components, and effector molecules in the control of New World cutaneous leishmaniasis due to L. braziliensis. Unlike L. major, L. braziliensis caused small, nonulcerative, and self-healing skin swelling in C57BL/6 mice, as well as BALB/c mice. In contrast to the results obtained for L. mexicana, mice deficient for interleukin-12 or its key signaling molecule, signal transducer and activator of transcription 4, rapidly succumbed to severe visceral leishmaniasis. Infection of tumor necrosis factor knockout mice with L. braziliensis led to progressive, nonhealing skin lesions with erosions and hemorrhagic ulcerations, but in contrast to the results with L. major, only 20 to 30% of the mice developed fatal visceral disease. As seen with L. major, mice with a deleted inducible nitric oxide synthase gene (iNOS(-/-)) were unable to contain L. braziliensis in the skin, whereas the control of the parasite in the spleen remained unimpaired. Unlike what happens in L. major infections, NADPH oxidase had no impact on the course of disease in L. braziliensis-infected mice. These results not only define essential components of a protective immune response to L. braziliensis but also illustrate that the requirements for the control of cutaneous leishmaniasis vary between different parasite species.
Figures
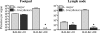

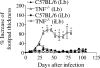
References
-
- Amato, V. S., H. F. Andrade, V. A. Neto, and M. I. S. Duarte. 2003. Persistence of tumor necrosis factor-α in situ after lesion healing in mucosal leishmaniasis. Am. J. Trop. Med. Hyg. 68:527-528. - PubMed
-
- Anderson, C. F., S. Mendez, and D. L. Sacks. 2005. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J. Immunol. 174:2934-2941. - PubMed
-
- Barral, A., J. Guerreiro, G. Bomfim, D. Correia, M. Barral-Netto, and E. M. Carvalho. 1995. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am. J. Trop. Med. Hyg. 53:256-259. - PubMed
-
- Belosevic, M., D. S. Finbloom, P. H. van der Meide, M. V. Slayter, and C. A. Nacy. 1989. Administration of monoclonal anti-IFN-γ antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J. Immunol. 143:266-274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

