Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection
- PMID: 17517871
- PMCID: PMC1951999
- DOI: 10.1128/IAI.00225-07
Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection
Abstract
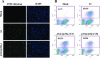
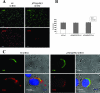
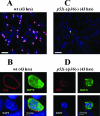
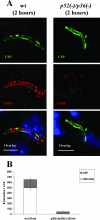
Malaria infection starts when sporozoites are transmitted to the mammalian host during a mosquito bite. Sporozoites enter the blood circulation, reach the liver, and infect hepatocytes. The formation of a parasitophorous vacuole (PV) establishes their intracellular niche. Recently, two members of the 6-Cys domain protein family, P52 and P36, were each shown to play an important albeit nonessential role in Plasmodium berghei sporozoite infectivity for the rodent host. Here, we generated p52/p36-deficient Plasmodium yoelii parasites by the simultaneous deletion of both genes using a single genetic manipulation. p52/p36-deficient parasites exhibited normal progression through the life cycle during blood-stage infection, transmission to mosquitoes, mosquito-stage development, and sporozoite infection of the salivary glands. p52/p36-deficient sporozoites also showed normal motility and cell traversal activity. However, immunofluorescence analysis and electron microscopic observations revealed that p52/p36-deficient parasites did not form a PV within hepatocytes in vitro and in vivo. The p52/p36-deficient parasites localized as free entities in the host cell cytoplasm or the host cell nucleoplasm and did not develop as liver stages. Consequently, they did not cause blood-stage infections even at high sporozoite inoculation doses. Mice immunized with p52/p36-deficient sporozoites were completely protected against infectious sporozoite challenge. Our results demonstrate for the first time the generation of two-locus gene deletion-attenuated parasites that infect the liver but do not progress to blood-stage infection. The study will critically guide the design of Plasmodium falciparum live attenuated malaria vaccines.
Figures


References
-
- Baer, K., M. Roosevelt, A. B. Clarkson, Jr., N. van Rooijen, T. Schnieder, and U. Frevert. 2007. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 9:397-412. - PubMed
-
- Belmonte, M., T. R. Jones, M. Lu, R. Arcilla, T. Smalls, A. Belmonte, J. Rosenbloom, D. J. Carucci, and M. Sedegah. 2003. The infectivity of Plasmodium yoelii in different strains of mice. J. Parasitol. 89:602-603. - PubMed
-
- Carter, R., A. Coulson, S. Bhatti, B. J. Taylor, and J. F. Elliott. 1995. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol. Biochem. Parasitol. 71:203-210. - PubMed
-
- Eksi, S., B. Czesny, G. J. van Gemert, R. W. Sauerwein, W. Eling, and K. C. Williamson. 2006. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 61:991-998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

