Mycolactone-mediated inhibition of tumor necrosis factor production by macrophages infected with Mycobacterium ulcerans has implications for the control of infection
- PMID: 17517872
- PMCID: PMC1951989
- DOI: 10.1128/IAI.00290-07
Mycolactone-mediated inhibition of tumor necrosis factor production by macrophages infected with Mycobacterium ulcerans has implications for the control of infection
Abstract
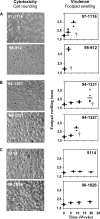
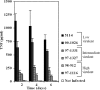
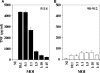
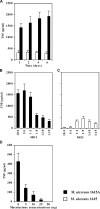
The pathogenicity of Mycobacterium ulcerans, the agent of Buruli ulcer, depends on the cytotoxic exotoxin mycolactone. Little is known about the immune response to this pathogen. Following the demonstration of an intracellular growth phase in the life cycle of M. ulcerans, we investigated the production of tumor necrosis factor (TNF) induced by intramacrophage bacilli of diverse toxigenesis/virulence, as well as the biological relevance of TNF during M. ulcerans experimental infections. Our data show that murine bone marrow-derived macrophages infected with mycolactone-negative strains of M. ulcerans (nonvirulent) produce high amounts of TNF, while macrophages infected with mycolactone-positive strains of intermediate or high virulence produce intermediate or low amounts of TNF, respectively. These results are in accordance with the finding that TNF receptor P55-deficient (TNF-P55 KO) mice are not more susceptible than wild-type mice to infection by the highly virulent strains but are more susceptible to nonvirulent and intermediately virulent strains, demonstrating that TNF is required to control the proliferation of these strains in animals experimentally infected by M. ulcerans. We also show that mycolactone produced by intramacrophage M. ulcerans bacilli inhibits, in a dose-dependent manner, but does not abrogate, the production of macrophage inflammatory protein 2, which is consistent with the persistent inflammatory responses observed in experimentally infected mice.
Figures

References
-
- Abalos, F. M., J. Aguiar, Sr., A. Guedenon, F. Portaels, and W. M. Meyers. 2000. Mycobacterium ulcerans infection (Buruli ulcer): a case report of the disseminated nonulcerative form. Ann. Diagn. Pathol. 4:386-390. - PubMed
-
- Adusumilli, S., A. Mve-Obiang, T. Sparer, W. Meyers, J. Hayman, and P. L. Small. 2005. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell. Microbiol. 7:1295-1304. - PubMed
-
- Asiedu, K., and S. Etuaful. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59:1015-1022. - PubMed
-
- Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. - PubMed
-
- Bendtzen, K. 1988. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol. Lett. 19:183-191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

