Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling
- PMID: 17517960
- PMCID: PMC2064204
- DOI: 10.1083/jcb.200611046
Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling
Abstract
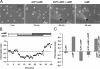
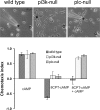
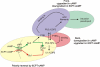
During embryonic development, cell movement is orchestrated by a multitude of attractants and repellents. Chemoattractants applied as a gradient, such as cAMP with Dictyostelium discoideum or fMLP with neutrophils, induce the activation of phospholipase C (PLC) and phosphoinositide 3 (PI3)-kinase at the front of the cell, leading to the localized depletion of phosphatidylinositol 4,5-bisphosphate (PI[4,5]P(2)) and the accumulation of phosphatidylinositol-3,4,5-trisphosphate (PI[3,4,5]P(3)). Using D. discoideum, we show that chemorepellent cAMP analogues induce localized inhibition of PLC, thereby reversing the polarity of PI(4,5)P(2). This leads to the accumulation of PI(3,4,5)P(3) at the rear of the cell, and chemotaxis occurs away from the source. We conclude that a PLC polarity switch controls the response to attractants and repellents.
Figures

References
-
- Affolter, M., and C.J. Weijer. 2005. Signaling to cytoskeletal dynamics during chemotaxis. Dev. Cell. 9:19–34. - PubMed
-
- Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature. 392:565–568. - PubMed
-
- Bominaar, A.A., and P.J.M. Van Haastert. 1993. Chemotactic antagonists of cAMP inhibit Dictyostelium phospholipase C. J. Cell Sci. 104:181–185. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

