Mice lacking NKCC1 are protected from development of bacteremia and hypothermic sepsis secondary to bacterial pneumonia
- PMID: 17517966
- PMCID: PMC2118609
- DOI: 10.1084/jem.20061205
Mice lacking NKCC1 are protected from development of bacteremia and hypothermic sepsis secondary to bacterial pneumonia
Abstract
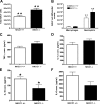
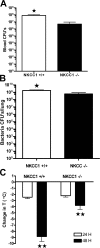
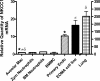
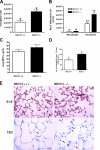
The contribution of the Na(+)-K(+)-Cl(-) transporter (NKCC1) to fluid in ion transport and fluid secretion in the lung and in other secretory epithelia has been well established. Far less is known concerning the role of this cotransporter in the physiological response of the pulmonary system during acute inflammation. Here we show that mice lacking this transporter are protected against hypothermic sepsis and bacteremia developing as a result of Klebsiella pneumoniae infection in the lung. In contrast, this protection was not observed in NKCC1(-/-) mice with K. pneumoniae-induced peritonitis. Although overall recruitment of cells to the lungs was not altered, the number of cells present in the airways was increased in the NKCC1(-/-) animals. Despite this robust inflammatory response, the increase in vascular permeability observed in this acute inflammatory model was attenuated in the NKCC1(-/-) animals. Our studies suggest that NKCC1 plays a unique and untoward unrecognized role in acute inflammatory responses in the lung and that specific inhibition of this NKCC isoform could be beneficial in treatment of sepsis.
Figures
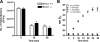


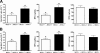

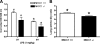
References
-
- Wheeler, A.P., and G.R. Bernard. 1999. Treating patients with severe sepsis. N. Engl. J. Med. 340:207–214. - PubMed
-
- Hudson, L.D., and K.P. Steinberg. 1999. Epidemiology of acute lung injury and ARDS. Chest. 116:74S–82S. - PubMed
-
- Hasleton, P.S., and T.E. Roberts. 1999. Adult respiratory distress syndrome—an update. Histopathology. 34:285–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

