Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering
- PMID: 17518596
- PMCID: PMC12019782
- DOI: 10.1089/ten.2006.0182
Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering
Abstract
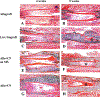
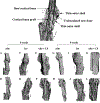
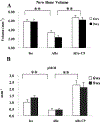
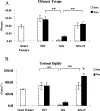
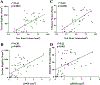
The presence of live periosteal progenitor cells on the surface of bone autografts confers better healing than devitalized allograft. We have previously demonstrated in a murine 4 mm segmental femoral bone-grafting model that live periosteum produces robust endochondral and intramembraneous bone formation that is essential for effective healing and neovascularization of structural bone grafts. To the end of engineering a live pseudo-periosteum that could induce a similar response onto devitalized bone allograft, we seeded a mesenchymal stem cell line stably transfected with human bone morphogenic protein-2/beta-galactosidase (C9) onto devitalized bone allografts or onto a membranous small intestinal submucosa scaffold that was wrapped around the allograft. Histology showed that C9-coated allografts displayed early cartilaginous tissue formation at day 7. By 6 and 9 weeks, a new cortical shell was found bridging the segmental defect that united the host bones. Biomechanical testing showed that C9-coated allografts displayed torsional strength and stiffness equivalent to intact femurs at 6 weeks and superior to live isografts at 9 weeks. Volumetric and histomorphometric micro-computed tomography analyses demonstrated a 2-fold increase in new bone formation around C9-coated allografts, which resulted in a substantial increase in polar moment of inertia (pMOI) due to the formation of new cortical shell around the allografts. Positive correlations between biomechanics and new bone volume and pMOI were found, suggesting that the biomechanical function of the grafted femur relates to both morphological parameters. C9-coated allograft also exhibited slower resorption of the graft cortex at 9 weeks than live isograft. Both new bone formation and the persistent allograft likely contributed to the improved biomechanics of C9-coated allograft. Taken together, we propose a novel strategy to combine structural bone allograft with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering.
Figures



References
-
- Burchardt H 1983. The biology of bone graft repair. Clin Orthop 28–42. - PubMed
-
- Burchardt H 1987. Biology of bone transplantation. Orthop Clin North Am 18:187–196. - PubMed
-
- Lord CF, Gebhardt MC, Tomford WW, and Mankin HJ 1988. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am 70:369–376. - PubMed
-
- Berrey BH Jr., Lord CF, Gebhardt MC, and Mankin HJ 1990. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am 72:825–833. - PubMed
-
- Enneking WF, and Mindell ER 1991. Observations on massive retrieved human allografts. J Bone Joint Surg Am 73:1123–1142. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

