Canonical Wnt signaling functions in second heart field to promote right ventricular growth
- PMID: 17519332
- PMCID: PMC1890492
- DOI: 10.1073/pnas.0701212104
Canonical Wnt signaling functions in second heart field to promote right ventricular growth
Abstract
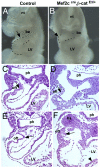
The second heart field (SHF), progenitor cells that are initially sequestered outside the heart, migrates into the heart and gives rise to endocardium, myocardium, and smooth muscle. Because of its distinct developmental history, the SHF is likely subjected to different signals from that of the first heart field. Previous experiments revealed that canonical Wnt signaling negatively regulated first heart field specification. We inactivated the obligate canonical Wnt effector beta-catenin using a beta-catenin conditional null allele and the Mef2c AHF cre driver that directs cre activity specifically in SHF. We also expressed a stabilized form of beta-catenin to model continuous Wnt signaling in SHF. Our data indicate that Wnt signaling acts in a positive fashion to promote right ventricular and interventricular myocardial expansion. Cyclin D2 and Tgfbeta2 expression was drastically reduced in beta-catenin loss-of-function mutants, indicating that Wnt signaling is required for patterning and expansion of SHF derivatives. Our findings reveal that Wnt signaling plays a major positive role in promoting growth and diversification of SHF precursors into right ventricular and interventricular myocardium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

