High-yield hydrogen production from starch and water by a synthetic enzymatic pathway
- PMID: 17520015
- PMCID: PMC1866174
- DOI: 10.1371/journal.pone.0000456
High-yield hydrogen production from starch and water by a synthetic enzymatic pathway
Abstract
Background: The future hydrogen economy offers a compelling energy vision, but there are four main obstacles: hydrogen production, storage, and distribution, as well as fuel cells. Hydrogen production from inexpensive abundant renewable biomass can produce cheaper hydrogen, decrease reliance on fossil fuels, and achieve zero net greenhouse gas emissions, but current chemical and biological means suffer from low hydrogen yields and/or severe reaction conditions.
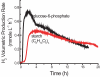
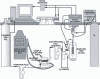
Methodology/principal findings: Here we demonstrate a synthetic enzymatic pathway consisting of 13 enzymes for producing hydrogen from starch and water. The stoichiometric reaction is C(6)H(10)O(5) (l)+7 H(2)O (l)-->12 H(2) (g)+6 CO(2) (g). The overall process is spontaneous and unidirectional because of a negative Gibbs free energy and separation of the gaseous products with the aqueous reactants.
Conclusions: Enzymatic hydrogen production from starch and water mediated by 13 enzymes occurred at 30 degrees C as expected, and the hydrogen yields were much higher than the theoretical limit (4 H(2)/glucose) of anaerobic fermentations.
Significance: The unique features, such as mild reaction conditions (30 degrees C and atmospheric pressure), high hydrogen yields, likely low production costs ($ approximately 2/kg H(2)), and a high energy-density carrier starch (14.8 H(2)-based mass%), provide great potential for mobile applications. With technology improvements and integration with fuel cells, this technology also solves the challenges associated with hydrogen storage, distribution, and infrastructure in the hydrogen economy.
Conflict of interest statement
Figures



References
-
- Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, et al. The path forward for biofuels and biomaterials. Science. 2006;311:484–489. - PubMed
-
- Morris D. The next economy: from dead carbon to living carbon. J Sci Food Agric. 2006;86:1743–1746.
-
- Hoffert MI, Caldeira K, Benford G, Criswell DR, Green C, et al. Advanced technology paths to global climate stability: energy for a greenhouse planet. Science. 2002;298:981–987. - PubMed
-
- Farrell AE, Plevin RJ, Turner BT, Jones AD, O'Hare M, et al. Ethanol can contribute to energy and environmental goals. Science. 2006;311:506–508. - PubMed
-
- DOE. Basic Research Needs for the Hydrogen Economy. 2004. http://www.sc.doe.gov/bes/hydrogen.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

